Team:Paris Saclay/Notebook/August/1
From 2013.igem.org
(Difference between revisions)
(→3 - Electrophoresis of the PCR products : RBS-BphR2 Part I) |
|||
| (10 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
===='''Objective : obtaining FNR and BphR2 proteins'''==== | ===='''Objective : obtaining FNR and BphR2 proteins'''==== | ||
| - | ===='''1 - Gel purification of PCR products : BphR2 Part I, BphR2 Part II, FNR Part I, FNR Part II, RBS-FNR Part I | + | ===='''1 - Gel purification of PCR products : BphR2 Part I, BphR2 Part II, FNR Part I, FNR Part II, RBS-FNR Part I in plasmid pSB1C3 '''==== |
Xavier | Xavier | ||
| Line 25: | Line 25: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | The PCR | + | The PCR products were good. Now we will do the Gibson assembly. |
|} | |} | ||
| Line 35: | Line 35: | ||
* BphR2 : | * BphR2 : | ||
| - | ** | + | ** Gibson PCR of pSB1C3 : 2µL |
** BphR2 Part I : 1µL | ** BphR2 Part I : 1µL | ||
** BphR2 Part II : 1µL | ** BphR2 Part II : 1µL | ||
| Line 42: | Line 42: | ||
* FNR : | * FNR : | ||
| - | ** | + | ** Gibson PCR of pSB1C3 : 2µL |
** FNR Part I : 1µL | ** FNR Part I : 1µL | ||
** FNR Part II : 1µL | ** FNR Part II : 1µL | ||
| Line 49: | Line 49: | ||
* RBS-FNR : | * RBS-FNR : | ||
| - | ** | + | ** Gibson PCR of pSB1C3: 2µL |
** RBS-FNR Part I : 1µL | ** RBS-FNR Part I : 1µL | ||
** FNR Part II : 1µL | ** FNR Part II : 1µL | ||
| Line 55: | Line 55: | ||
** H2O : 1µL | ** H2O : 1µL | ||
| - | We | + | We incubated these Gibson assembly mixes at 50°C during 1h inside PCR machine. |
| - | ===='''3 - PCR of | + | ===='''3 - PCR of RBS-BphR2 Part I'''==== |
Abdou | Abdou | ||
| Line 66: | Line 66: | ||
** Oligo 55R : 1µL | ** Oligo 55R : 1µL | ||
** Buffer phusion : 5µL | ** Buffer phusion : 5µL | ||
| - | ** DNA : 0.25µL | + | ** DNA (''P. pseudoalcaligenes'' KF 707 genomic DNA) : 0.25µL |
| - | ** dNTP : 1µL | + | ** dNTP 10mM : 1µL |
| - | ** Phusion : 5µL | + | ** Enzyme Phusion : 5µL |
** H2O : 36.5µL | ** H2O : 36.5µL | ||
| Line 80: | Line 80: | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |File: | + | | style="width:350px;border:1px solid black;" |[[File:Psgel10108.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL of DNA Ladder | * Well 1 : 6µL of DNA Ladder | ||
| - | * Well 2 : 5µL of RBS-BphR2 Part I+1µl of 6X loading dye | + | * Well 2 : 5µL of RBS-BphR2 Part I + 1µl of 6X loading dye |
* Gel : 0.8% | * Gel : 0.8% | ||
|} | |} | ||
Expected sizes : | Expected sizes : | ||
| - | * RBS-BphR2 Part I : | + | * RBS-BphR2 Part I : 197pb |
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained our fragment at the right size. We will purify it. |
|} | |} | ||
Latest revision as of 17:08, 3 October 2013
Notebook : August 1
Lab work
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
Objective : obtaining FNR and BphR2 proteins
1 - Gel purification of PCR products : BphR2 Part I, BphR2 Part II, FNR Part I, FNR Part II, RBS-FNR Part I in plasmid pSB1C3
Xavier
Protocol : Gel purification
Nanodrop :
- BphR2 Part I : 44 ng/µL
- BphR2 Part II : 64 ng/µL
- FNR Part I : 147 ng/µL
- FNR Part II : 140 ng/µL
- RBS-FNR Part I : 167 ng/µL
- PSB1C3 : 159 ng/µL
|
The PCR products were good. Now we will do the Gibson assembly. |
2 - Gibson assembly.
Abdou, Xiaojing
Used quantities :
- BphR2 :
- Gibson PCR of pSB1C3 : 2µL
- BphR2 Part I : 1µL
- BphR2 Part II : 1µL
- Gibson mix : 15µL
- H2O : 1µL
- FNR :
- Gibson PCR of pSB1C3 : 2µL
- FNR Part I : 1µL
- FNR Part II : 1µL
- Gisbon mix : 15µL
- H2O : 1µL
- RBS-FNR :
- Gibson PCR of pSB1C3: 2µL
- RBS-FNR Part I : 1µL
- FNR Part II : 1µL
- Gibson mix : 15µL
- H2O : 1µL
We incubated these Gibson assembly mixes at 50°C during 1h inside PCR machine.
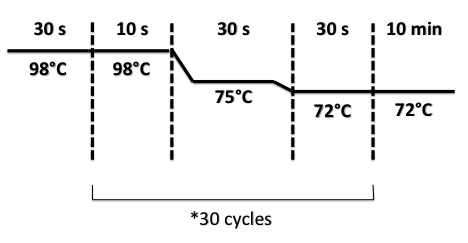
3 - PCR of RBS-BphR2 Part I
Abdou
Used quantities :
- Oligo 54F : 1µL
- Oligo 55R : 1µL
- Buffer phusion : 5µL
- DNA (P. pseudoalcaligenes KF 707 genomic DNA) : 0.25µL
- dNTP 10mM : 1µL
- Enzyme Phusion : 5µL
- H2O : 36.5µL
PCR Program :
3 - Electrophoresis of the PCR products : RBS-BphR2 Part I
Xavier, XiaoJing
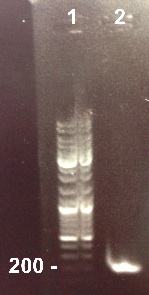
|
|
Expected sizes :
- RBS-BphR2 Part I : 197pb
|
We obtained our fragment at the right size. We will purify it. |
| Previous day | Back to calendar | Next day |
 "
"