Team:Paris Saclay/Notebook/August/8
From 2013.igem.org
| Line 25: | Line 25: | ||
*GFP : 1069kb | *GFP : 1069kb | ||
| - | + | {| | |
| - | + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | |
| - | + | We obtain fragments at the right size. We will purify the highest band which contains the PSB3K3 plasmid. | |
| + | |} | ||
| - | + | ====2 - Electrophoresis of Bba_J004450 digested by EcoRI/PstI==== | |
Anaïs | Anaïs | ||
| Line 42: | Line 43: | ||
{| | {| | ||
| - | | style="border:1px solid black;padding:5px;background-color:# | + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | |
We obtain fragments at the right size. We will purify the highest band which contains the PSB3K3 plasmid. | We obtain fragments at the right size. We will purify the highest band which contains the PSB3K3 plasmid. | ||
|} | |} | ||
| - | + | ==== 3- Electroelution of PSB3K3 digested by EcoRI/PstI==== | |
Nadia | Nadia | ||
| Line 53: | Line 54: | ||
We let the plasmid precipitate during the night. | We let the plasmid precipitate during the night. | ||
| + | |||
| + | ===='''Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006'''==== | ||
| + | |||
| + | ===='''1 - Tranduction of Km in MG1655Z1 ==== | ||
| + | |||
| + | Anaïs, Nadia | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | We didn't obtain colonies from the transduction of 08/07/13. We will do it again. | ||
| + | |} | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/Protocols/transduction|Transduction]] | ||
| + | |||
| + | Our Mutant bacteria was called BW : Δfnr::Km. | ||
| + | Our wild type bacteria was called MG1655Z1. | ||
| + | |||
| + | We did the first step of the protocol : bacteriophage stock which packed Km gene. | ||
| + | |||
===='''Objective : obtaining Bba_K1155007'''==== | ===='''Objective : obtaining Bba_K1155007'''==== | ||
Revision as of 14:34, 28 September 2013
Notebook : August 8
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining the PSB3K3 backbone plasmid
1 - Electrophoresis of BBa_J004450 digested by EcoRI/Pst1 to check if the digestion
Damir, Nadia
| IMAGE |
|
Expected sizes :
- PSB3K3 : 2750kb
- GFP : 1069kb
|
We obtain fragments at the right size. We will purify the highest band which contains the PSB3K3 plasmid. |
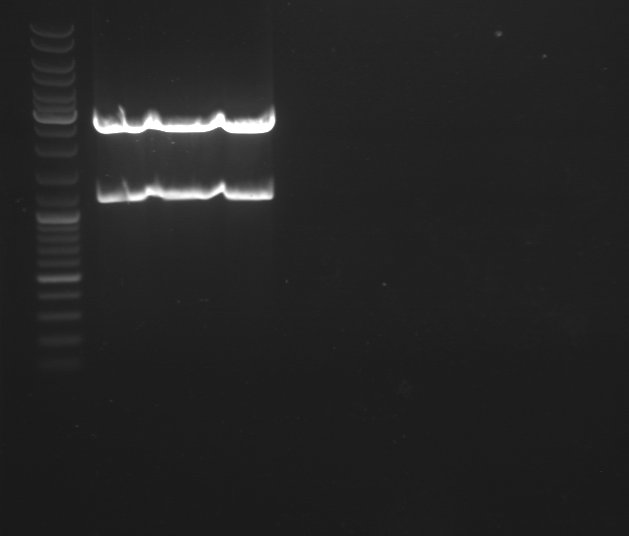
2 - Electrophoresis of Bba_J004450 digested by EcoRI/PstI
Anaïs
|
|
|
We obtain fragments at the right size. We will purify the highest band which contains the PSB3K3 plasmid. |
3- Electroelution of PSB3K3 digested by EcoRI/PstI
Nadia
Protocol : Electroelution
We let the plasmid precipitate during the night.
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Tranduction of Km in MG1655Z1
Anaïs, Nadia
|
We didn't obtain colonies from the transduction of 08/07/13. We will do it again. |
Protocol : Transduction
Our Mutant bacteria was called BW : Δfnr::Km. Our wild type bacteria was called MG1655Z1.
We did the first step of the protocol : bacteriophage stock which packed Km gene.
Objective : obtaining Bba_K1155007
1 - Colony PCR of Bba_K115007 in DH5α
Anaïs
|
Tranformation of 08/07/13 works. we will make a PCR Colony. |
Colonie repiquée dans 10µL d'eau pour chaque tube ???????
Used quantities :
- DNA : 2µL
- Mix : (it was divided in 25 tubes for each promotor with 23µL of mix in each on)
- Oligo ... : 3.5µL
- Oligo ... : 3.5µL
- Buffer Dream Taq : 70µL
- dNTP : 28µL
- Dream Taq : 5µL
- H2O : 590µL
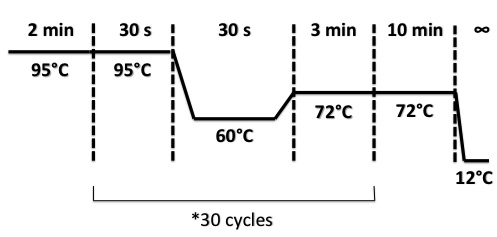
PCR Program :
2 - Electrophoresis to check the colony PCR products : Bba_K1155007
Anaïs, Damir
| 350px |
|
Expected size :
- Bba_K1155007 : 3583 bp
|
We obtain fragment at the right size for colonies 10, 14 and 15. We will extract BBa_K1155007. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Extraction of plasmid of BphR2, FNR, RBS-FNR
Damir
|
Transformation of 08/02/13 works. We will extract plasmids. |
Protocol : Gel purification
|
We lost our plasmids. We will do the Gibson assembly again. |
2 - Gel purification of RBS-BphR2 Part I
Nadia, XiaoJing
Protocol : Gel purification
|
We lost fragment. We will do the PCR again. |
| Previous day | Back to calendar | Next day |
 "
"