Team:Paris Saclay/Notebook/August/9
From 2013.igem.org
(Difference between revisions)
(Created page with "{{Team:Paris_Saclay/incl_debut_generique}} ='''Notebook : August 9'''= =='''Lab work'''== ==='''A - Aerobic/Anaerobic regulation system'''=== ===='''Obtaining Δ ''fnr E. col...") |
(→1 - Tranduction of Km in MG1655Z1) |
||
| (28 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ====''' | + | ===='''Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
| - | ==== | + | ===='''1 - Tranduction of Km in MG1655Z1 ==== |
| - | Damir, Nadia | + | |
| + | Abdou, Anaïs, Damir, Nadia, XiaoJing | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | | + | | style="border:1px solid black;padding:5px;background-color:#DE;" | |
| + | We observed lysis areas in the strain MG1655Z1 Δfnr::Km after transduction. We will continue the transduction protocol. | ||
| + | |} | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" | [[File:Ps908transduction.jpg|350px]] | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | + | '''Picture: lysed cells comparison.''' | |
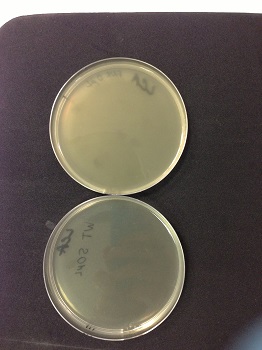
| - | * | + | * 0µl phage(control): the petri dish is cloudy, bacteria are not lysed. |
| - | * | + | * 50µl phage: the petri dish is clear, bacteria are lysed by phages. |
|} | |} | ||
| - | + | ===='''Objective : obtaining BBa_K1155007'''==== | |
| - | + | ====1 - Extraction of BBa_K115007 from DH5αstrain==== | |
| - | + | ||
| - | + | Abdou | |
| - | + | Protocol : [[Team:Paris_Saclay/extraction|High-copy plamid extraction]] | |
| - | + | We extracted plamid from colonies number 10, 14 and 15. | |
| - | + | Nanodrop | |
| + | * BBa_K1155007 in clone 10 : 38ng/µl | ||
| + | * BBa_K1155007 in clone 14 : 48.5ng/µl | ||
| + | * BBa_K1155007 in clone 15 : 52 ng/µl | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" | [[File: | + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | |
| + | The extraction was good. We will sequence our plasmids. | ||
| + | |} | ||
| + | |||
| + | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensing system'''=== | ||
| + | |||
| + | ====Objective : Obtaining FNR and BphR2 proteins==== | ||
| + | |||
| + | ===='''1 - Electrophoresis of the PCR of BphR2 Part I, BphR2 Part II, RBS_BphR2 Part I, FNR Part I, FNR Part II, RBS_FNR Part I to check the gel purification'''==== | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel10908.jpg]] | ||
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
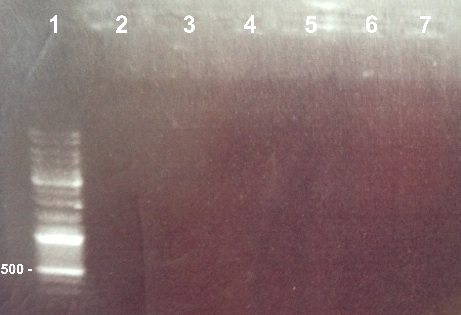
*Well 1 : 6µL DNA Ladder | *Well 1 : 6µL DNA Ladder | ||
| - | *Well 2 : | + | *Well 2 : 5µL of BphR2 Part I + 1µl of 6X loading dye |
| + | *Well 3 : 5µL of BphR2 Part II + 1µl of 6X loading dye | ||
| + | *Well 4 : 5µL of RBS-BphR2 Part I + 1µl of 6X loading dye | ||
| + | *Well 5 : 5µL of FNR Part I + 1µl of 6X loading dye | ||
| + | *Well 6 : 5µL of FRN Part II + 1µl of 6X loading dye | ||
| + | *Well 7: 5µL of RBS-FNR Part I + 1µl of 6X loading dye | ||
*Gel : 0.8% | *Gel : 0.8% | ||
|} | |} | ||
| - | + | Expected size : | |
| + | * BphR2 Part I : 178 bp | ||
| + | * BphR2 Part II : 790bp | ||
| + | * RBS-BphR2 Part I : 197bp | ||
| + | * FNR Part I : 597 bp | ||
| + | * FNR Part II : 200bp | ||
| + | * RBS-FNR PartI : 615bp | ||
| - | + | {| | |
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We lost all our PCR fragments. We will do the PCR again. | ||
| + | |} | ||
| - | + | ===='''2 - PCR of BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I, FNR Part I, FNR Part II, RBS-FNR Part I'''==== | |
| - | + | Anaïs, Damir, Nadia, XiaoJing | |
| - | + | Used quantities : | |
| + | * Bphr2 Part I : | ||
| + | ** Oligo 54F : 1µL | ||
| + | ** Oligo 55R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA of ''Pseudomonas pseudoalcaligenes'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| - | + | * Bphr2 Part II : | |
| + | ** Oligo 56F : 1µL | ||
| + | ** Oligo 57R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Pseudomonas pseudoalcaligenes'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| - | + | * RBS-Bphr2 Part I : | |
| - | + | ** Oligo 58F : 1µL | |
| + | ** Oligo 57R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Pseudomonas pseudoalcaligenes'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| - | * | + | * FNR Part I : |
| - | ** | + | ** Oligo 59F : 1µL |
| - | ** | + | ** Oligo 60R : 1µL |
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Escherichia coli'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| - | * | + | * FNR Part II : |
| + | ** Oligo 61F : 1µL | ||
| + | ** Oligo 62R : 1µL | ||
| + | ** Buffer Phusion : 10µL | ||
| + | ** DNA ''Escherichia coli'' : 1µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Phusion : 0.5µL | ||
| + | ** H2O : 35.5µL | ||
| - | * | + | * RBS-FNR Part I : |
| - | ** | + | ** Oligo 63F : 1µL |
| - | ** | + | ** Oligo 62R : 1µL |
| - | ** | + | ** Buffer Phusion : 10µL |
| - | ** | + | ** DNA ''Escherichia coli'' : 1µL |
| - | ** | + | ** dNTP : 1µL |
| - | ** | + | ** Phusion : 0.5µL |
| + | ** H2O : 35.5µL | ||
| - | + | PCR Program : | |
| - | + | * BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I : | |
| - | + | ||
| - | + | [[File:PsPCRBphR23007.jpg|400px]] | |
| - | + | ||
| - | + | * FNR Part I, FNR Part II, RBS-FNR Part I : | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[File:PsPCRFNR3007.jpg|400px]] | |
| - | + | ||
| + | {| border="1" align="center" | ||
| + | |[[Team:Paris Saclay/Notebook/August/8|<big>Previous day</big>]] | ||
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/August/12|<big>Next day</big>]] | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 15:28, 4 October 2013
Notebook : August 9
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Tranduction of Km in MG1655Z1
Abdou, Anaïs, Damir, Nadia, XiaoJing
|
We observed lysis areas in the strain MG1655Z1 Δfnr::Km after transduction. We will continue the transduction protocol. |
|
Picture: lysed cells comparison.
|
Objective : obtaining BBa_K1155007
1 - Extraction of BBa_K115007 from DH5αstrain
Abdou
Protocol : High-copy plamid extraction
We extracted plamid from colonies number 10, 14 and 15.
Nanodrop
- BBa_K1155007 in clone 10 : 38ng/µl
- BBa_K1155007 in clone 14 : 48.5ng/µl
- BBa_K1155007 in clone 15 : 52 ng/µl
|
The extraction was good. We will sequence our plasmids. |
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
Objective : Obtaining FNR and BphR2 proteins
1 - Electrophoresis of the PCR of BphR2 Part I, BphR2 Part II, RBS_BphR2 Part I, FNR Part I, FNR Part II, RBS_FNR Part I to check the gel purification
Expected size :
- BphR2 Part I : 178 bp
- BphR2 Part II : 790bp
- RBS-BphR2 Part I : 197bp
- FNR Part I : 597 bp
- FNR Part II : 200bp
- RBS-FNR PartI : 615bp
|
We lost all our PCR fragments. We will do the PCR again. |
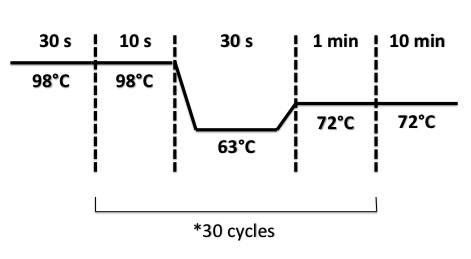
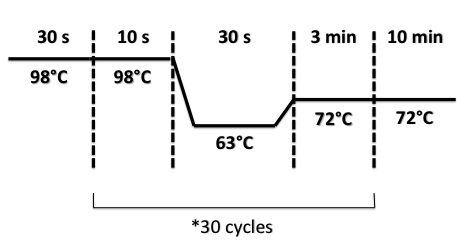
2 - PCR of BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I, FNR Part I, FNR Part II, RBS-FNR Part I
Anaïs, Damir, Nadia, XiaoJing
Used quantities :
- Bphr2 Part I :
- Oligo 54F : 1µL
- Oligo 55R : 1µL
- Buffer Phusion : 10µL
- DNA of Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- Bphr2 Part II :
- Oligo 56F : 1µL
- Oligo 57R : 1µL
- Buffer Phusion : 10µL
- DNA Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- RBS-Bphr2 Part I :
- Oligo 58F : 1µL
- Oligo 57R : 1µL
- Buffer Phusion : 10µL
- DNA Pseudomonas pseudoalcaligenes : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part I :
- Oligo 59F : 1µL
- Oligo 60R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- FNR Part II :
- Oligo 61F : 1µL
- Oligo 62R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
- RBS-FNR Part I :
- Oligo 63F : 1µL
- Oligo 62R : 1µL
- Buffer Phusion : 10µL
- DNA Escherichia coli : 1µL
- dNTP : 1µL
- Phusion : 0.5µL
- H2O : 35.5µL
PCR Program :
- BphR2 Part I, BphR2 Part II, RBS-BphR2 Part I :
- FNR Part I, FNR Part II, RBS-FNR Part I :
| Previous day | Back to calendar | Next day |
 "
"