Team:Paris Saclay/Notebook/July/3
From 2013.igem.org
(→1 - Colony PCR of BBa_K1155000 to check good insertion of Pndh* in pSB1C3) |
(→1 - Colony PCR of BBa_K1155000 to check good insertion of Pndh* in pSB1C3) |
||
| (3 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DE;" | | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| - | Tranformation of 07/02/13 works. We will do a | + | Tranformation of 07/02/13 works. We will do a PCR colony. |
|} | |} | ||
| Line 21: | Line 21: | ||
* Standard concentration : | * Standard concentration : | ||
| - | ** BBa_K1155000 : 0 | + | ** BBa_K1155000 : 0 colony |
* High concentration : | * High concentration : | ||
| - | ** BBa_K1155000 : 2 | + | ** BBa_K1155000 : 2 colonies |
[[File:Ps0307jour.jpg|300px]] | [[File:Ps0307jour.jpg|300px]] | ||
| Line 30: | Line 30: | ||
<center>[[File:PSprimer07.jpg|right|250px]]</center> | <center>[[File:PSprimer07.jpg|right|250px]]</center> | ||
<br> | <br> | ||
| - | '''[Primers] and PCR :''' | + | '''[https://2013.igem.org/Team:Paris_Saclay/Primers Primers] and PCR :''' |
<p>'''VF2, VR, Pfnr_up, Pfnr_down are four oligos that we used for plasmid amplification. We used tree combinaisons VF/VR, VF/Pfnr_Down, Pfnr_Up/VR.''' | <p>'''VF2, VR, Pfnr_up, Pfnr_down are four oligos that we used for plasmid amplification. We used tree combinaisons VF/VR, VF/Pfnr_Down, Pfnr_Up/VR.''' | ||
| - | '''If the promoter | + | '''If the promoter Pndh* insert pSB1C3 plasmid successfully, tree fragments with specific size will be amplified.''' </p> |
| + | ''' | ||
| + | Protocol''' | ||
| - | We | + | We resuspend each colony with 20µL of H2O. |
Used quantities : | Used quantities : | ||
| Line 40: | Line 42: | ||
* Mix : (it was divided in tubes for 4 different colonies for each oligo combinaison with 23µL of mix in each tube) | * Mix : (it was divided in tubes for 4 different colonies for each oligo combinaison with 23µL of mix in each tube) | ||
** VF or Pfnr_Up : 6µL | ** VF or Pfnr_Up : 6µL | ||
| - | ** VR or Pfnr_Down | + | ** VR or Pfnr_Down : 6µL |
| - | ** dNTP : 6µL | + | ** dNTP 10mM : 6µL |
** Buffer Dream Taq : 30µL | ** Buffer Dream Taq : 30µL | ||
** Dream Taq : 6µL | ** Dream Taq : 6µL | ||
| Line 53: | Line 55: | ||
We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_K1155000. We also did liquid culture of each one. | We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_K1155000. We also did liquid culture of each one. | ||
| - | We | + | We incubate culture at 37°C. |
===='''Objective : obtaining BBa_K1155003, BBa_K1155007'''==== | ===='''Objective : obtaining BBa_K1155003, BBa_K1155007'''==== | ||
| Line 67: | Line 69: | ||
We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_I732017, BBa_K592009. We also did liquid culture of each one. | We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_I732017, BBa_K592009. We also did liquid culture of each one. | ||
| - | We | + | We incubate culture at 37°C. |
Latest revision as of 00:51, 5 October 2013
Contents |
Notebook : July 3
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155000
1 - Colony PCR of BBa_K1155000 to check good insertion of Pndh* in pSB1C3
Abdou, Sheng, Zhou
|
Tranformation of 07/02/13 works. We will do a PCR colony. |
Colonies count for BBa_K1155000 :
- Standard concentration :
- BBa_K1155000 : 0 colony
- High concentration :
- BBa_K1155000 : 2 colonies
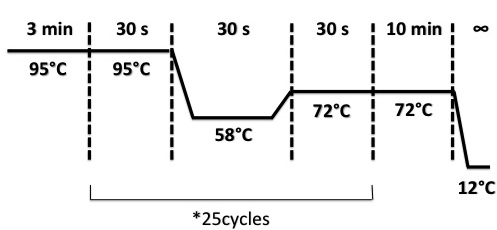
Primers and PCR :
VF2, VR, Pfnr_up, Pfnr_down are four oligos that we used for plasmid amplification. We used tree combinaisons VF/VR, VF/Pfnr_Down, Pfnr_Up/VR. If the promoter Pndh* insert pSB1C3 plasmid successfully, tree fragments with specific size will be amplified.
Protocol
We resuspend each colony with 20µL of H2O.
Used quantities :
- DNA : 2µL
- Mix : (it was divided in tubes for 4 different colonies for each oligo combinaison with 23µL of mix in each tube)
- VF or Pfnr_Up : 6µL
- VR or Pfnr_Down : 6µL
- dNTP 10mM : 6µL
- Buffer Dream Taq : 30µL
- Dream Taq : 6µL
- H2O : 246µL
2 - Culture of BBa_K1155000
Sheng
We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_K1155000. We also did liquid culture of each one. We incubate culture at 37°C.
Objective : obtaining BBa_K1155003, BBa_K1155007
1 - Culture of BBa_I732017, BBa_K592009
Sheng
|
Tranformation of 07/02/13 works. We will do new cultures. |
We made new cultures by streaking 2 colonies of 07/02/13 culture of BBa_I732017, BBa_K592009. We also did liquid culture of each one. We incubate culture at 37°C.
| Previous day | Back to calendar | Next day |
 "
"