Team:Warsaw/Journal
From 2013.igem.org
| (3 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
====1st week: 2.07.13 – 5.07.13==== | ====1st week: 2.07.13 – 5.07.13==== | ||
| - | |||
Preparation of competent cells for chemical-based transfection. Failure. | Preparation of competent cells for chemical-based transfection. Failure. | ||
| - | |||
| - | |||
====2nd week: 8.07.13 – 12.07.13==== | ====2nd week: 8.07.13 – 12.07.13==== | ||
| - | |||
Succeed preparation of competent cells for chemical-based transfection. Verification with iGEM’s transformation kit. | Succeed preparation of competent cells for chemical-based transfection. Verification with iGEM’s transformation kit. | ||
Preparation of competent cells for electroporation. Failure. | Preparation of competent cells for electroporation. Failure. | ||
| - | |||
| - | |||
====3rd week: 15.07.13 – 19.07.13==== | ====3rd week: 15.07.13 – 19.07.13==== | ||
| - | |||
Chemical-based transformation. BioBricks: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2020, E2030. Failure: E2020. Succeed: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2030. | Chemical-based transformation. BioBricks: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2020, E2030. Failure: E2020. Succeed: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2030. | ||
Isolate plasmid DNA by alkaline lysis. Quality cheked with spectrophotomeric analysis with NanoDrop. | Isolate plasmid DNA by alkaline lysis. Quality cheked with spectrophotomeric analysis with NanoDrop. | ||
| - | |||
| - | |||
====4th week: 22.07.13 – 26.07.13==== | ====4th week: 22.07.13 – 26.07.13==== | ||
| - | |||
Transformation with isolated pladmids and BioBricks: I74609 and K864100. Isolate plasmid DNA (from I73609 and K864100) with mini-prep kit. Digest and ligation of construct: J23100 and B0034 on plasmid pSB1C3. | Transformation with isolated pladmids and BioBricks: I74609 and K864100. Isolate plasmid DNA (from I73609 and K864100) with mini-prep kit. Digest and ligation of construct: J23100 and B0034 on plasmid pSB1C3. | ||
| - | |||
| - | |||
====5th week: 29.07.13 – 1.08.13==== | ====5th week: 29.07.13 – 1.08.13==== | ||
| - | |||
Preparing construct: J23100 + B0034 + K864100 on plasmid pSB1A3. Measure glowing fluorescent proteins: sfGTP, sfYFP, sfCFP, sfBFP and SYFP2. Superfolded (sf) forms are made by Warsaw Team. SYFP2 is from parts registry. | Preparing construct: J23100 + B0034 + K864100 on plasmid pSB1A3. Measure glowing fluorescent proteins: sfGTP, sfYFP, sfCFP, sfBFP and SYFP2. Superfolded (sf) forms are made by Warsaw Team. SYFP2 is from parts registry. | ||
| - | |||
| - | |||
====6th week: 5.08.13 – 9.08.13===== | ====6th week: 5.08.13 – 9.08.13===== | ||
| - | |||
Measure glowing fluorescent proteins. Banking strains with our fluorescent proteins. | Measure glowing fluorescent proteins. Banking strains with our fluorescent proteins. | ||
| - | |||
| - | |||
====7th week: 12.08.13 – 14.08.13==== | ====7th week: 12.08.13 – 14.08.13==== | ||
| - | |||
Transformation BioBrick: E2050. | Transformation BioBrick: E2050. | ||
PCR: sfBFP N-terminal, sfYFP N-terminal and mCherry (J06504) C-terminal (succeed). Isolate plasmid DNA with: sfGFP (I73609), mOrange (E2050), sfCFP, sfYFP and sfBFP. | PCR: sfBFP N-terminal, sfYFP N-terminal and mCherry (J06504) C-terminal (succeed). Isolate plasmid DNA with: sfGFP (I73609), mOrange (E2050), sfCFP, sfYFP and sfBFP. | ||
| - | |||
| - | |||
====8th week: 19.08.13 – 23.08.13==== | ====8th week: 19.08.13 – 23.08.13==== | ||
| - | |||
PCR: sfGFP N-terminal (failure), sfCFP N-terminal (succeed) and mCherry N-terminal (succeed). | PCR: sfGFP N-terminal (failure), sfCFP N-terminal (succeed) and mCherry N-terminal (succeed). | ||
Gel-out: sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal. | Gel-out: sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal. | ||
Cloning sfCFP N-terminal, mCherry N-terminal, sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal to pSB1C3 plasmid and inserting it to bacteria. | Cloning sfCFP N-terminal, mCherry N-terminal, sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal to pSB1C3 plasmid and inserting it to bacteria. | ||
Preparing construct: J23100+B0034+E0040 – failure. | Preparing construct: J23100+B0034+E0040 – failure. | ||
| + | ====9th week: 26.08.13 – 30.08.13==== | ||
| + | '''Verification cloning''' | ||
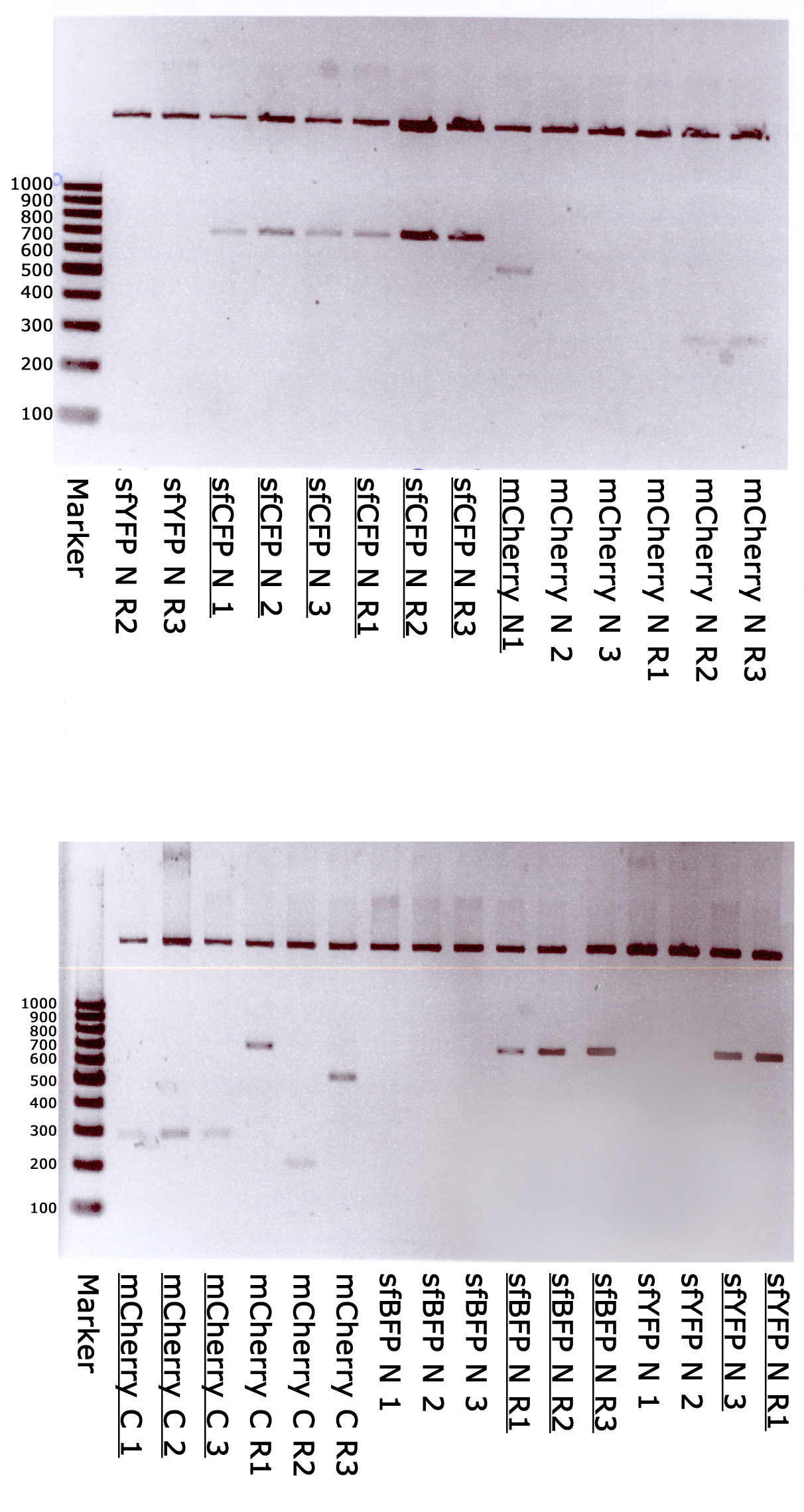
| - | + | [[File:Klonowanie.jpg|600px|border|caption]] | |
| - | + | Marker - GeneRuler 100 bp DNA Ladder, ready-to-use (Thermo); sfYFP N 1,2,3,R1,R2,R3 - random clones of sfYFP N-terminal; sfCFP N 1,2,3,R1,R2,R3 - random clones of sfCFP N-terminal; mCherry N 1,2,3,R1,R2,R3 - random clones of mCherry N-terminal; mCherry C 1,2,3,R1,R2,R3 - random clones of mCherry C-terminal; sfBFP N 1,2,3,R1,R2,R3 - random clones of sfBFP N-terminal. <u>Underlined</u> - correct clones. | |
| - | + | ||
| - | + | ||
| - | + | ||
And great success! | And great success! | ||
| - | |||
PCR: sfGFP N-terminal and GFP C-terminal (various attenuation template DNA) and sfC-terminal (to versions: m6 and m12 to all of superfolded fluorescent proteins). Cloning and inserting it to bacteria. | PCR: sfGFP N-terminal and GFP C-terminal (various attenuation template DNA) and sfC-terminal (to versions: m6 and m12 to all of superfolded fluorescent proteins). Cloning and inserting it to bacteria. | ||
Once again preparing construct: J23100+B0034+E0040. | Once again preparing construct: J23100+B0034+E0040. | ||
| - | |||
====10th week: 2.09.13 – 7.09.13==== | ====10th week: 2.09.13 – 7.09.13==== | ||
| - | |||
Welcome school! | Welcome school! | ||
Verification cloning. Unfortunately epic fail. | Verification cloning. Unfortunately epic fail. | ||
PCR: sfGFP N-terminal, GFP N-terminal and GFP C-terminal. At least success! | PCR: sfGFP N-terminal, GFP N-terminal and GFP C-terminal. At least success! | ||
| - | |||
====11th week: 10.09.13 – 14.09.13==== | ====11th week: 10.09.13 – 14.09.13==== | ||
| - | |||
Cloning sfGFP N-terminal, GFP N-terminal and GFP C-terminal. Unfortunately fail. Checking buffers for digesting – they’re fine. Searching mistakes in cloning. | Cloning sfGFP N-terminal, GFP N-terminal and GFP C-terminal. Unfortunately fail. Checking buffers for digesting – they’re fine. Searching mistakes in cloning. | ||
| - | |||
====12th week: 16.09.13 – 20.09.13==== | ====12th week: 16.09.13 – 20.09.13==== | ||
| - | |||
We get out commission from GeneRay. Cloning this to pSB1C3 plasmids, but it ends with failure. Cleaning lab and end work. | We get out commission from GeneRay. Cloning this to pSB1C3 plasmids, but it ends with failure. Cleaning lab and end work. | ||
| - | |||
{{:Team:Warsaw/Templates/StandardPageEnd}} | {{:Team:Warsaw/Templates/StandardPageEnd}} | ||
Latest revision as of 18:22, 1 October 2013
Genetic lab journal
1st week: 2.07.13 – 5.07.13
Preparation of competent cells for chemical-based transfection. Failure.
2nd week: 8.07.13 – 12.07.13
Succeed preparation of competent cells for chemical-based transfection. Verification with iGEM’s transformation kit. Preparation of competent cells for electroporation. Failure.
3rd week: 15.07.13 – 19.07.13
Chemical-based transformation. BioBricks: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2020, E2030. Failure: E2020. Succeed: J06504, J23100, B0034, I712074, E0040, B0015, B0017, E2030. Isolate plasmid DNA by alkaline lysis. Quality cheked with spectrophotomeric analysis with NanoDrop.
4th week: 22.07.13 – 26.07.13
Transformation with isolated pladmids and BioBricks: I74609 and K864100. Isolate plasmid DNA (from I73609 and K864100) with mini-prep kit. Digest and ligation of construct: J23100 and B0034 on plasmid pSB1C3.
5th week: 29.07.13 – 1.08.13
Preparing construct: J23100 + B0034 + K864100 on plasmid pSB1A3. Measure glowing fluorescent proteins: sfGTP, sfYFP, sfCFP, sfBFP and SYFP2. Superfolded (sf) forms are made by Warsaw Team. SYFP2 is from parts registry.
6th week: 5.08.13 – 9.08.13=
Measure glowing fluorescent proteins. Banking strains with our fluorescent proteins.
7th week: 12.08.13 – 14.08.13
Transformation BioBrick: E2050. PCR: sfBFP N-terminal, sfYFP N-terminal and mCherry (J06504) C-terminal (succeed). Isolate plasmid DNA with: sfGFP (I73609), mOrange (E2050), sfCFP, sfYFP and sfBFP.
8th week: 19.08.13 – 23.08.13
PCR: sfGFP N-terminal (failure), sfCFP N-terminal (succeed) and mCherry N-terminal (succeed). Gel-out: sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal. Cloning sfCFP N-terminal, mCherry N-terminal, sfYFP N-terminal, sfBFP N-terminal and mCherry C-terminal to pSB1C3 plasmid and inserting it to bacteria. Preparing construct: J23100+B0034+E0040 – failure.
9th week: 26.08.13 – 30.08.13
Verification cloning
Marker - GeneRuler 100 bp DNA Ladder, ready-to-use (Thermo); sfYFP N 1,2,3,R1,R2,R3 - random clones of sfYFP N-terminal; sfCFP N 1,2,3,R1,R2,R3 - random clones of sfCFP N-terminal; mCherry N 1,2,3,R1,R2,R3 - random clones of mCherry N-terminal; mCherry C 1,2,3,R1,R2,R3 - random clones of mCherry C-terminal; sfBFP N 1,2,3,R1,R2,R3 - random clones of sfBFP N-terminal. Underlined - correct clones.
And great success! PCR: sfGFP N-terminal and GFP C-terminal (various attenuation template DNA) and sfC-terminal (to versions: m6 and m12 to all of superfolded fluorescent proteins). Cloning and inserting it to bacteria. Once again preparing construct: J23100+B0034+E0040.
10th week: 2.09.13 – 7.09.13
Welcome school! Verification cloning. Unfortunately epic fail. PCR: sfGFP N-terminal, GFP N-terminal and GFP C-terminal. At least success!
11th week: 10.09.13 – 14.09.13
Cloning sfGFP N-terminal, GFP N-terminal and GFP C-terminal. Unfortunately fail. Checking buffers for digesting – they’re fine. Searching mistakes in cloning.
12th week: 16.09.13 – 20.09.13
We get out commission from GeneRay. Cloning this to pSB1C3 plasmids, but it ends with failure. Cleaning lab and end work.
 "
"