Team:Warsaw/Safety
From 2013.igem.org
Tosterovic (Talk | contribs) |
Annamiscicka (Talk | contribs) (→Part 1) |
||
| (12 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
| - | Here we present answers to questions | + | Here we present answers to questions asked in safety forms. |
| Line 47: | Line 47: | ||
| BBa_K1093000 | | BBa_K1093000 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 53: | Line 53: | ||
| BBa_K1093001 | | BBa_K1093001 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 59: | Line 59: | ||
| BBa_K1093002 | | BBa_K1093002 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 65: | Line 65: | ||
| BBa_K1093003 | | BBa_K1093003 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 71: | Line 71: | ||
| BBa_K1093004 | | BBa_K1093004 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 77: | Line 77: | ||
| BBa_K1093005 | | BBa_K1093005 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 83: | Line 83: | ||
| BBa_K1093006 | | BBa_K1093006 | ||
| PartsRegistry BBa_I746909 | | PartsRegistry BBa_I746909 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 89: | Line 89: | ||
| BBa_K1093007 | | BBa_K1093007 | ||
| oligo.pl (complementary oligonucleotides) | | oligo.pl (complementary oligonucleotides) | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 95: | Line 95: | ||
| BBa_K1093008 | | BBa_K1093008 | ||
| oligo.pl (complementary oligonucleotides) | | oligo.pl (complementary oligonucleotides) | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 101: | Line 101: | ||
| BBa_K1093009 | | BBa_K1093009 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 107: | Line 107: | ||
| BBa_K1093010 | | BBa_K1093010 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 113: | Line 113: | ||
| BBa_K1093011 | | BBa_K1093011 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 119: | Line 119: | ||
| BBa_K1093012 | | BBa_K1093012 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 125: | Line 125: | ||
| BBa_K1093013 | | BBa_K1093013 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria, Homo sapiens | + | | ''Aequorea victoria, Homo sapiens'' |
| 1 | | 1 | ||
| fluorescence/gluthatione-dependent redox enzyme | | fluorescence/gluthatione-dependent redox enzyme | ||
| Line 131: | Line 131: | ||
| BBa_K1093013 | | BBa_K1093013 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Aequorea victoria, Escherichia coli | + | | ''Aequorea victoria, Escherichia coli'' |
| 1 | | 1 | ||
| fluorescence/ gluthatione-dependent redox enzyme | | fluorescence/ gluthatione-dependent redox enzyme | ||
| Line 137: | Line 137: | ||
| BBa_K1093014 | | BBa_K1093014 | ||
| PartsRegistry BBa_E0040 | | PartsRegistry BBa_E0040 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 143: | Line 143: | ||
| BBa_K1093015 | | BBa_K1093015 | ||
| PartsRegistry BBa_E0040 | | PartsRegistry BBa_E0040 | ||
| - | | Aequorea victoria | + | | ''Aequorea victoria'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 149: | Line 149: | ||
| BBa_K1093016 | | BBa_K1093016 | ||
| PartsRegistry BBa_J06505 | | PartsRegistry BBa_J06505 | ||
| - | | Discosoma sp. | + | | ''Discosoma sp.'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 155: | Line 155: | ||
| BBa_K1093017 | | BBa_K1093017 | ||
| PartsRegistry BBa_J06505 | | PartsRegistry BBa_J06505 | ||
| - | | Discosoma sp. | + | | ''Discosoma sp.'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 161: | Line 161: | ||
| BBa_K1093018 | | BBa_K1093018 | ||
| PartsRegistry BBa_E2050 | | PartsRegistry BBa_E2050 | ||
| - | | Discosoma sp. | + | | ''Discosoma sp.'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 167: | Line 167: | ||
| BBa_K1093019 | | BBa_K1093019 | ||
| PartsRegistry BBa_E2050 | | PartsRegistry BBa_E2050 | ||
| - | | Discosoma sp. | + | | ''Discosoma sp.'' |
| 1 | | 1 | ||
| fluorescence | | fluorescence | ||
| Line 173: | Line 173: | ||
| BBa_K1093020 | | BBa_K1093020 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens, Aequorea victoria | + | | ''Homo sapiens, Aequorea victoria'' |
| 1 | | 1 | ||
| oxygen transport | | oxygen transport | ||
| Line 179: | Line 179: | ||
| BBa_K1093021 | | BBa_K1093021 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens, Aequorea victoria | + | | ''Homo sapiens, Aequorea victoria'' |
| 1 | | 1 | ||
| oxygen transport | | oxygen transport | ||
| Line 185: | Line 185: | ||
| BBa_K1093022 | | BBa_K1093022 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens | + | | ''Homo sapiens'' |
| 1 | | 1 | ||
| oxygen transport | | oxygen transport | ||
| Line 191: | Line 191: | ||
| BBa_K1093023 | | BBa_K1093023 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Escherichia coli, bacteriophage T4 | + | | ''Escherichia coli'', bacteriophage T4 |
| 1 | | 1 | ||
| lysis/cell division | | lysis/cell division | ||
| Line 197: | Line 197: | ||
| BBa_K1093024 | | BBa_K1093024 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Escherichia coli, bacteriophage T4, Vibrio fisheri | + | | ''Escherichia coli'', bacteriophage T4, ''Vibrio fisheri'' |
| 1 | | 1 | ||
| lysis/cell division/quorum sensing | | lysis/cell division/quorum sensing | ||
| Line 203: | Line 203: | ||
| BBa_K1093025 | | BBa_K1093025 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Escherichia coli | + | | ''Escherichia coli'' |
| 1 | | 1 | ||
| cell division | | cell division | ||
| Line 209: | Line 209: | ||
| BBa_K1093026 | | BBa_K1093026 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens | + | | ''Homo sapiens'' |
| 1 | | 1 | ||
| transcription factor | | transcription factor | ||
| Line 215: | Line 215: | ||
| BBa_K1093027 | | BBa_K1093027 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens | + | | ''Homo sapiens'' |
| 1 | | 1 | ||
| transcription factor | | transcription factor | ||
| Line 221: | Line 221: | ||
| BBa_K1093028 | | BBa_K1093028 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens | + | | ''Homo sapiens'' |
| 1 | | 1 | ||
| transcription factor | | transcription factor | ||
| Line 227: | Line 227: | ||
| BBa_K1093029 | | BBa_K1093029 | ||
| Synthesized, GeneRay | | Synthesized, GeneRay | ||
| - | | Homo sapiens | + | | ''Homo sapiens'' |
| 1 | | 1 | ||
| oxygen transport | | oxygen transport | ||
| Line 269: | Line 269: | ||
'''a. Please provide a link to your institution biosafety guidelines.''' | '''a. Please provide a link to your institution biosafety guidelines.''' | ||
| - | Our | + | Our institution does not have any specific biosafety guidelines.It is using the ones present at the Faculty of Biology or the one present in the Institute of Biochemistry and Biophysics. |
'''b. Does your institution have an Institutional Biosafety Committee, or an equivalent group? If yes, have you discussed your project with them? Describe any concerns they raised with your project, and any changes you made to your project plan based on their review.''' | '''b. Does your institution have an Institutional Biosafety Committee, or an equivalent group? If yes, have you discussed your project with them? Describe any concerns they raised with your project, and any changes you made to your project plan based on their review.''' | ||
| - | There is no group of this kind at our institution. | + | There is no group of this kind at our institution.But our project has been discussed with many experienced researchers that know well biosafety and they didn't raise any concerns. |
'''c. Does your country have national biosafety regulations or guidelines? If so, please provide a link to these regulations or guidelines if possible.''' | '''c. Does your country have national biosafety regulations or guidelines? If so, please provide a link to these regulations or guidelines if possible.''' | ||
| Line 308: | Line 308: | ||
'''4. How did you physically acquire the organism or part?''' | '''4. How did you physically acquire the organism or part?''' | ||
| - | + | We have obtained the cell lines from the Institute of Genetics and Biotechnology. | |
'''5. What potential safety/health risks to team members, other people at your institution, or the general public could arise from your use of this organism/part?''' | '''5. What potential safety/health risks to team members, other people at your institution, or the general public could arise from your use of this organism/part?''' | ||
| - | None. Mammalian cells that | + | None. Mammalian cells that we are using are not harmful for people and environment. |
'''6. What measures do you intend to take to ensure that your project is safe for team members, other people at your institution, and the general public?''' | '''6. What measures do you intend to take to ensure that your project is safe for team members, other people at your institution, and the general public?''' | ||
| Line 324: | Line 324: | ||
'''8. Why do you need to use this organism/part? Is there an organism/part from a less dangerous Risk Group that would accomplish the same purpose?''' | '''8. Why do you need to use this organism/part? Is there an organism/part from a less dangerous Risk Group that would accomplish the same purpose?''' | ||
| - | We are using different cell lines to demonstrate cytotoxic | + | We are using different cell lines to demonstrate cytotoxic effects of acrylamide. As our work with |
mammalian cells is absolutely safe, there is no need to search for another solutions. | mammalian cells is absolutely safe, there is no need to search for another solutions. | ||
'''9. Is the organism/part listed under the Australia Group guidelines, or otherwise restricted for transport? If so, how will your team ship this part to iGEM and the Jamborees?''' | '''9. Is the organism/part listed under the Australia Group guidelines, or otherwise restricted for transport? If so, how will your team ship this part to iGEM and the Jamborees?''' | ||
| - | We are not going to ship the cell lines to iGEM. | + | We are not going to ship the cell lines to iGEM. We will only present the outcomes on the Jamborees. |
'''10. Please describe the BioSafety Level of the lab in which the team works, or description of safety features of lab (Refer to Basic Safety form, question 8. d.). If you are using organisms with a BSL level greater than you lab, please explain any additional safety precautions you are taking.''' | '''10. Please describe the BioSafety Level of the lab in which the team works, or description of safety features of lab (Refer to Basic Safety form, question 8. d.). If you are using organisms with a BSL level greater than you lab, please explain any additional safety precautions you are taking.''' | ||
| Line 339: | Line 339: | ||
* placing materials to be decontaminated outside the cell lab in a durable, leak proof containers | * placing materials to be decontaminated outside the cell lab in a durable, leak proof containers | ||
* using disposable glassware | * using disposable glassware | ||
| - | * | + | * careful management of glassware, especially broken pipettes must be handled carefully |
| + | |||
| + | |||
| + | ==== Part 3 ==== | ||
| + | |||
| + | '''Kill switches: description''' | ||
| + | |||
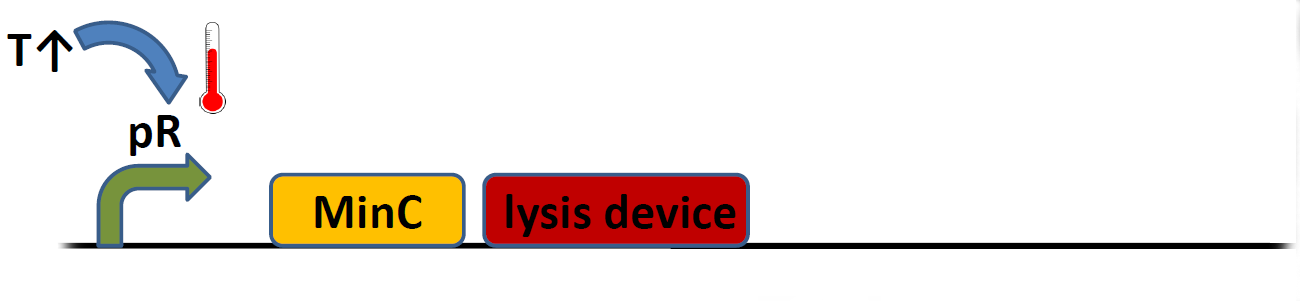
| + | Lysozyme/holin-based lysis device (BBa_K112808) has been described in detail. While it is highly efficient in releasing proteins into the medium (thus making it ideal for our hemoglobin-based sensor), its creators concluded it did not meet their expectations as a kill switch, since a fraction of bacterial population usually adapted and was selected for following the device induction. Our initial idea was to improve upon this design by adding MinC protein to the system (cell division inhibitor, initialy tested by Warsaw 2010 Team). This brute force approach can be summarised by a simple pictorial: | ||
| + | |||
| + | [[File:Kill direct.png|700px|thumb|center|alt=A|Kill switch - the simple version BBa_K1093023]] | ||
| + | |||
| + | We soon began to consider alternative solutions. Wouldn't it be better to inhibit cell division before inducing the lysis (and thus applying strong selective pressure against the effective expression of lysis device)? After all, MinC doee not inhibit bacterial growth or DNA replication, but it does prevent division. Thus even a few copies of kill switch without deleterious mutations preserved within the long filamentous bacterium should be sufficient to cause cell death. This reasoning lead us to the following design: | ||
| + | |||
| + | [[File:Kill delayed.png|700px|thumb|center|alt=A|Kill switch - the version with delayed lysis BBa_K1093024]] | ||
| + | |||
| + | This design applies LuxI/LuxR-based countdown device in order to separate the expression of MinC and lysis device. Eventually, we decided to test both approaches. | ||
| + | |||
| + | Due to their size, synthesis of complete constructs would not be practical, both financially and timewise. The sequence of the simpler version was divided into two subparts. The delayed version was divided into five smaller fragments. Despite this effort, we only received two fragments of our kill switch prior to wiki freeze. | ||
| + | |||
| + | |||
| + | |||
| + | '''Mathematical model of the kill switches''' | ||
| + | |||
| + | [[File:Warsaw_Kill_swich_1.png|thumb|left|alt=A|Kill switch 1 - kinetic differential equations]] | ||
| + | [[File:Warsaw_Kill_swich_2.png|thumb|rigth|alt=A|Kill switch 2 - kinetic differential equations]] | ||
| + | |||
| + | |||
| + | The behavior of any genetic circuit can be described using ordinary differential equations (ODE) formalism. In our case we decided to applied ODE due to its relative simplicity. In this approximation a genetic circuit is treat similarly to a set of simple chemical reactions. | ||
| + | |||
| + | The first kill switch is relatively simple. Because the expression of repressor protein (Repr) is regulated by a constitutive promoter (pRepr), when temperature is 30 C its concentration is constant, hence expression of MinC, lysis protein 1 (LD1) and lysis protein 2 (LD2) is negligible. However, if the temperature is raised to 42 C the repressor is rapidly inactivated, which results in elevated expression of MinC, lysis protein 1 and lysis protein 2 which leads to cell disruption. | ||
| + | |||
| + | The second kill switch is more complicated since it contains the so called "countdown device" based on quorum sensing system. In this circuit LuxR and Repr are under a constitutive promoter. In normal temperature an inactive transcription factor LuxR is expressed. On the other hand, LuxI (which expression is controlled by the pR promoter, along with MinC) is an enzyme capable of converting S-adenosyl-cysteine (PreM) to an active mediator (M), which acts as transcription factor upon binding M. The activated LuxR binds to pL promoter and this leads to expression of both lysis protein. | ||
| + | |||
| + | |||
| + | |||
| + | |||
{{:Team:Warsaw/Templates/StandardPageEnd}} | {{:Team:Warsaw/Templates/StandardPageEnd}} | ||
Latest revision as of 03:19, 5 October 2013
Safety
Contents |
Here we present answers to questions asked in safety forms.
Part 1
Please describe the chassis organism(s) you will be using for this project. If you will be using more than one chassis organism, provide information on each of them:
| Species | Strain | Risk group | Risk group soruce link | Disease risk to humans |
| E. coli | DH5 | 1 | www.absa.org/riskgroups/bacteriasearch.php?genus=&species=coli | Yes. May cause irritation to skin, eyes, and respiratory tract, may affect kidneys. |
| E. coli | BL21 | 1 | www.absa.org/riskgroups/bacteriasearch.php?genus=&species=coli | Yes. May cause irritation to skin, eyes, and respiratory tract, may affect kidneys. |
| E. coli | TOP10 | 1 | www.absa.org/riskgroups/bacteriasearch.php?genus=&species=coli | Yes. May cause irritation to skin, eyes, and respiratory tract, may affect kidneys. |
3. List and describe all new or modified coding regions you will be using in your project. (If you use parts from the 2013 iGEM Distribution without modifying them, you do not need to list those parts.)
| Part number. | Where did you get the physical DNA for this part (which lab, synthesis company, etc) | What species does this part originally come from? | What is the Risk Group of the species? | What is the function of this part, in its parent species? |
| BBa_K1093000 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093001 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093002 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093003 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093004 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093005 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093006 | PartsRegistry BBa_I746909 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093007 | oligo.pl (complementary oligonucleotides) | Aequorea victoria | 1 | fluorescence |
| BBa_K1093008 | oligo.pl (complementary oligonucleotides) | Aequorea victoria | 1 | fluorescence |
| BBa_K1093009 | Synthesized, GeneRay | Aequorea victoria | 1 | fluorescence |
| BBa_K1093010 | Synthesized, GeneRay | Aequorea victoria | 1 | fluorescence |
| BBa_K1093011 | Synthesized, GeneRay | Aequorea victoria | 1 | fluorescence |
| BBa_K1093012 | Synthesized, GeneRay | Aequorea victoria | 1 | fluorescence |
| BBa_K1093013 | Synthesized, GeneRay | Aequorea victoria, Homo sapiens | 1 | fluorescence/gluthatione-dependent redox enzyme |
| BBa_K1093013 | Synthesized, GeneRay | Aequorea victoria, Escherichia coli | 1 | fluorescence/ gluthatione-dependent redox enzyme |
| BBa_K1093014 | PartsRegistry BBa_E0040 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093015 | PartsRegistry BBa_E0040 | Aequorea victoria | 1 | fluorescence |
| BBa_K1093016 | PartsRegistry BBa_J06505 | Discosoma sp. | 1 | fluorescence |
| BBa_K1093017 | PartsRegistry BBa_J06505 | Discosoma sp. | 1 | fluorescence |
| BBa_K1093018 | PartsRegistry BBa_E2050 | Discosoma sp. | 1 | fluorescence |
| BBa_K1093019 | PartsRegistry BBa_E2050 | Discosoma sp. | 1 | fluorescence |
| BBa_K1093020 | Synthesized, GeneRay | Homo sapiens, Aequorea victoria | 1 | oxygen transport |
| BBa_K1093021 | Synthesized, GeneRay | Homo sapiens, Aequorea victoria | 1 | oxygen transport |
| BBa_K1093022 | Synthesized, GeneRay | Homo sapiens | 1 | oxygen transport |
| BBa_K1093023 | Synthesized, GeneRay | Escherichia coli, bacteriophage T4 | 1 | lysis/cell division |
| BBa_K1093024 | Synthesized, GeneRay | Escherichia coli, bacteriophage T4, Vibrio fisheri | 1 | lysis/cell division/quorum sensing |
| BBa_K1093025 | Synthesized, GeneRay | Escherichia coli | 1 | cell division |
| BBa_K1093026 | Synthesized, GeneRay | Homo sapiens | 1 | transcription factor |
| BBa_K1093027 | Synthesized, GeneRay | Homo sapiens | 1 | transcription factor |
| BBa_K1093028 | Synthesized, GeneRay | Homo sapiens | 1 | transcription factor |
| BBa_K1093029 | Synthesized, GeneRay | Homo sapiens | 1 | oxygen transport |
4. Do the biological materials used in your lab work pose any of the following risks? Please describe.
a. Risks to the safety and health of team members or others working in the lab?
Laboratory strains of E. coli are not pathogenic, thus they do not pose threat for anyone in the lab.
b. Risks to the safety and health of the general public, if released by design or by accident?
None of the biological materials we are working with this year can pose threat to safety and health of genral public.
c. Risks to the environment, if released by design or by accident?
The only materials possibly hazardous to the environment we are working with are plasmids containing genes coding resistance for antibiotics such as ampicillin, chloramphenicol and kanamycin.
d. Risks to security through malicious misuse by individuals, groups, or countries?
There is no possibility of malicious misuse.
5. If your project moved from a small-scale lab study to become widely used as a commercial/industrial product, what new risks might arise? (Consider the different categories of risks that are listed in parts a-d of the previous question.) Also, what risks might arise if the knowledge you generate or the methods you develop became widely available? (Note: This is meant to be a somewhat open-ended discussion question.)
Our project is not meant for public use in open environment. If our acrylamide sensor became widely used as a commercial product its area of usage would still be laboratories and other closed facilities. None of the biological parts, methods and ideas we developed introduce novelties that can be directly misused and therefore pose a risk to anyone.
6. Does your project include any design features to address safety risks? (For example: kill switches, auxotrophic chassis, etc.) Note that including such features is not mandatory to participate in iGEM, but many groups choose to include them.
Our project includes a kill switch. It is based on two components: MinC protein (which inhibits cell division) and T4 bateriophage derived lysis device. MinC expression will be induced firstly, to reduce the odds of bacteria adapting via negative selection. Expression of the lysis device will be delayed with the application of LuXI/LuxR timer device.
7. What safety training have you received (or plan to receive in the future)? Provide a brief description, and a link to your institution’s safety training requirements, if available.
As all students of University of Warsaw we received standard training in area of hygienic and safe work with special emphasis on matters connected to our area of study.
8. Under what biosafety provisions will / do you work?
We are working accoriding to the guidelines for conducting work with GMO issued by Polish government.
a. Please provide a link to your institution biosafety guidelines.
Our institution does not have any specific biosafety guidelines.It is using the ones present at the Faculty of Biology or the one present in the Institute of Biochemistry and Biophysics.
b. Does your institution have an Institutional Biosafety Committee, or an equivalent group? If yes, have you discussed your project with them? Describe any concerns they raised with your project, and any changes you made to your project plan based on their review.
There is no group of this kind at our institution.But our project has been discussed with many experienced researchers that know well biosafety and they didn't raise any concerns.
c. Does your country have national biosafety regulations or guidelines? If so, please provide a link to these regulations or guidelines if possible.
Yes, we do have such regulations: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20010760811.
d. According to the WHO Biosafety Manual, what is the BioSafety Level rating of your lab? (Check the summary table on page 3, and the fuller description that starts on page 9.) If your lab does not fit neatly into category 1, 2, 3, or 4, please describe its safety features [see 2013.igem.org/Safety for help].
1
e. What is the Risk Group of your chassis organism(s), as you stated in question 1? If it does not match the BSL rating of your laboratory, please explain what additional safety measures you are taking.
The risk group of organisms is 1.
Part 2
1. Organism name and strain name or number.
Following mammalian cell lines:
- HEK 293
- HeLa
- 143 B
- SH SY5Y
2. Organism Risk Group:
As mammalian cells are not classified as microorganisms, they are not included in the ABSA Risk Group list
3. If you are using this organism as a chassis, write "chassis". If you are using a genetic part from the organism, give the name of the part and a brief description of what it does and why you are using it.
None of the above. See paragraph 8.
4. How did you physically acquire the organism or part?
We have obtained the cell lines from the Institute of Genetics and Biotechnology.
5. What potential safety/health risks to team members, other people at your institution, or the general public could arise from your use of this organism/part?
None. Mammalian cells that we are using are not harmful for people and environment.
6. What measures do you intend to take to ensure that your project is safe for team members, other people at your institution, and the general public?
As our work in the cell lab is safe and unharmful to others there is no need to undertake additional safety measurements. What we will be working on in our project is to check the cytotoxity of acrylamide. Our aim is to show how acrylamide may affect different tissues reflected by the cell lines derived from these tissues. We would like to examine how different concentrations of acrylamide may influence the cells and draw a parallel to living organisms.
7. If you are using only a part from the organism, and you believe the part by itself is not dangerous, explain why you believe it is not dangerous.
Question not relevant.
8. Why do you need to use this organism/part? Is there an organism/part from a less dangerous Risk Group that would accomplish the same purpose?
We are using different cell lines to demonstrate cytotoxic effects of acrylamide. As our work with mammalian cells is absolutely safe, there is no need to search for another solutions.
9. Is the organism/part listed under the Australia Group guidelines, or otherwise restricted for transport? If so, how will your team ship this part to iGEM and the Jamborees?
We are not going to ship the cell lines to iGEM. We will only present the outcomes on the Jamborees.
10. Please describe the BioSafety Level of the lab in which the team works, or description of safety features of lab (Refer to Basic Safety form, question 8. d.). If you are using organisms with a BSL level greater than you lab, please explain any additional safety precautions you are taking.
In our cell lab we obey all of the safety rules provided. Our supervisor familiarized us with the safety rules that are as follows:
- wearing aprons and gloves while working in the lab
- not drinking or eating in the lab
- decontamination of stocks, and other materials before disposal, using an effective method.
- placing materials to be decontaminated outside the cell lab in a durable, leak proof containers
- using disposable glassware
- careful management of glassware, especially broken pipettes must be handled carefully
Part 3
Kill switches: description
Lysozyme/holin-based lysis device (BBa_K112808) has been described in detail. While it is highly efficient in releasing proteins into the medium (thus making it ideal for our hemoglobin-based sensor), its creators concluded it did not meet their expectations as a kill switch, since a fraction of bacterial population usually adapted and was selected for following the device induction. Our initial idea was to improve upon this design by adding MinC protein to the system (cell division inhibitor, initialy tested by Warsaw 2010 Team). This brute force approach can be summarised by a simple pictorial:
We soon began to consider alternative solutions. Wouldn't it be better to inhibit cell division before inducing the lysis (and thus applying strong selective pressure against the effective expression of lysis device)? After all, MinC doee not inhibit bacterial growth or DNA replication, but it does prevent division. Thus even a few copies of kill switch without deleterious mutations preserved within the long filamentous bacterium should be sufficient to cause cell death. This reasoning lead us to the following design:
This design applies LuxI/LuxR-based countdown device in order to separate the expression of MinC and lysis device. Eventually, we decided to test both approaches.
Due to their size, synthesis of complete constructs would not be practical, both financially and timewise. The sequence of the simpler version was divided into two subparts. The delayed version was divided into five smaller fragments. Despite this effort, we only received two fragments of our kill switch prior to wiki freeze.
Mathematical model of the kill switches
The behavior of any genetic circuit can be described using ordinary differential equations (ODE) formalism. In our case we decided to applied ODE due to its relative simplicity. In this approximation a genetic circuit is treat similarly to a set of simple chemical reactions.
The first kill switch is relatively simple. Because the expression of repressor protein (Repr) is regulated by a constitutive promoter (pRepr), when temperature is 30 C its concentration is constant, hence expression of MinC, lysis protein 1 (LD1) and lysis protein 2 (LD2) is negligible. However, if the temperature is raised to 42 C the repressor is rapidly inactivated, which results in elevated expression of MinC, lysis protein 1 and lysis protein 2 which leads to cell disruption.
The second kill switch is more complicated since it contains the so called "countdown device" based on quorum sensing system. In this circuit LuxR and Repr are under a constitutive promoter. In normal temperature an inactive transcription factor LuxR is expressed. On the other hand, LuxI (which expression is controlled by the pR promoter, along with MinC) is an enzyme capable of converting S-adenosyl-cysteine (PreM) to an active mediator (M), which acts as transcription factor upon binding M. The activated LuxR binds to pL promoter and this leads to expression of both lysis protein.
 "
"