Team:Bielefeld-Germany/Modelling/Oxidation
From 2013.igem.org
(Created page with "{{Team:Bielefeld-Germany/Header2}} {{Team:Bielefeld-Germany/css/header_cleanup.css}} {{Team:Bielefeld-Germany/css/button.css}} __NOTOC__ <html> <style> h1{margin-top:70px; } ...") |
m |
||
| (41 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{Team:Bielefeld-Germany/css/header_cleanup.css}} | {{Team:Bielefeld-Germany/css/header_cleanup.css}} | ||
{{Team:Bielefeld-Germany/css/button.css}} | {{Team:Bielefeld-Germany/css/button.css}} | ||
| - | |||
| - | |||
__NOTOC__ | __NOTOC__ | ||
| Line 49: | Line 47: | ||
<h1>Modelling - Mediator Oxidation</h1> | <h1>Modelling - Mediator Oxidation</h1> | ||
| - | |||
<div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:10px; clear:both;"> | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:10px; clear:both;"> | ||
| Line 63: | Line 60: | ||
<p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Optimal">Optimal<br> conditions</a></p></div> | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Optimal">Optimal<br> conditions</a></p></div> | ||
<div class="bigbutton"> | <div class="bigbutton"> | ||
| - | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling | + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Two_Reactions">Two<br> Reactions</a></p></div> |
</div> | </div> | ||
</html> | </html> | ||
| - | == | + | ==Mediator Oxidation== |
<p align="justify"> | <p align="justify"> | ||
| - | In | + | In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation: <br><br> |
| - | + | ||
| - | + | [[File:Bielefeld-germany-model-oxid-reaction-1.PNG|300px|center]] | |
| - | |||
<br> | <br> | ||
| - | + | ||
| + | ,where M<sub>red</sub> is the reduced mediator and <br> | ||
| + | M<sub>ox</sub> the oxidized mediator. | ||
<br> | <br> | ||
| - | + | Then the current output can be calculated based on formula according to the Faraday's law:<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[File:Bielefeld-germany-model-oxid-reaction-2.PNG|250px|center]] | |
| - | [[ | + | <br><br> |
| + | ,where I is the current density [A]<br> | ||
| + | [M<sub>red</sub>] is the concentration of reduced mediator in the chamber<br> | ||
| + | ''n'' is the number of electrons taking part in the electrode reaction,<br> | ||
| + | F is the Faradays constant (96 500 C) and <br> | ||
| + | k<sub>3</sub> is the reaction rate of the mediator oxidation at the anode.<br> | ||
| + | </p> | ||
| + | <br> | ||
| + | ==Simulation== | ||
| + | <p align="justify"> | ||
| + | The value ''k<sub>3</sub>'' obtained as mentioned above was applied in the simulation of the current output in the final reaction of the three-reaction model. The start concentrations of the NAD<sup>+</sup> and oxidized mediator were set as in the simulations preformed for the proceeding reactions at 100 µM. The simulation time span was set for 60 sec. | ||
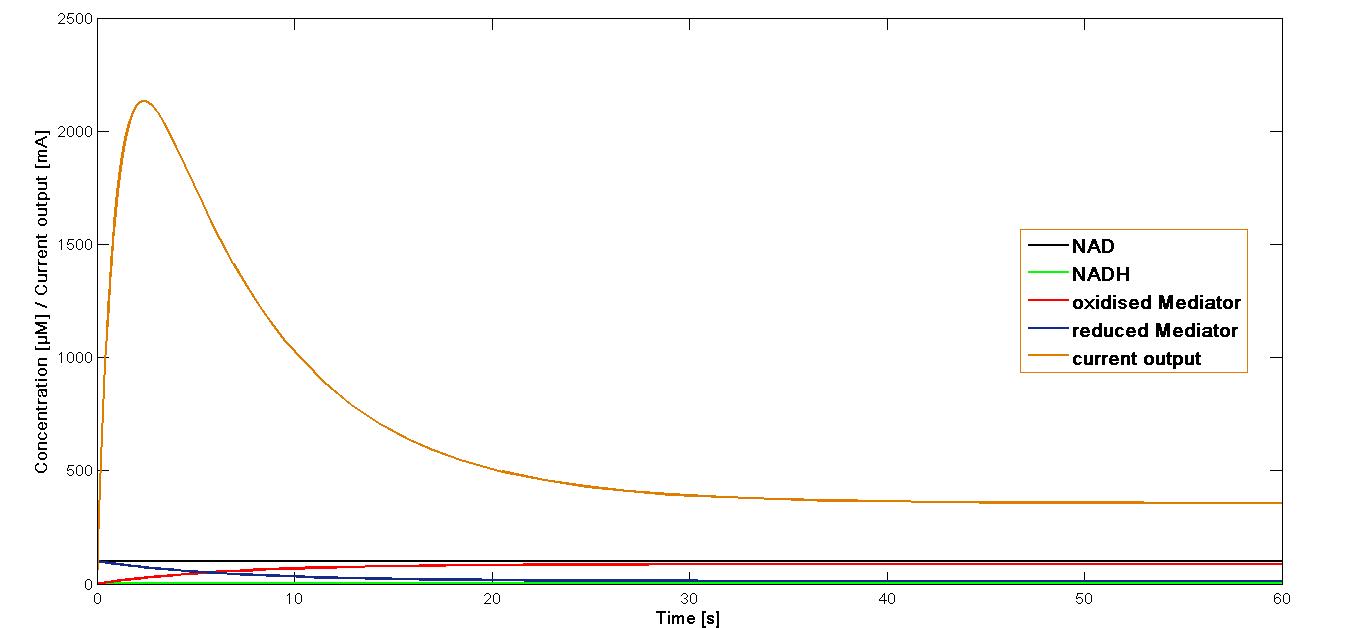
| + | The resulting plot for the mediator '''MB''' is shown in the Figure 1, below: | ||
| + | <br><br> | ||
| + | [[File:Bielefeld-germany-model-oxid-diagramm-MB-01.png|600px|center|thumb|'''Figure 1''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''MB''' at 100 µM.]] | ||
| + | <br><br> | ||
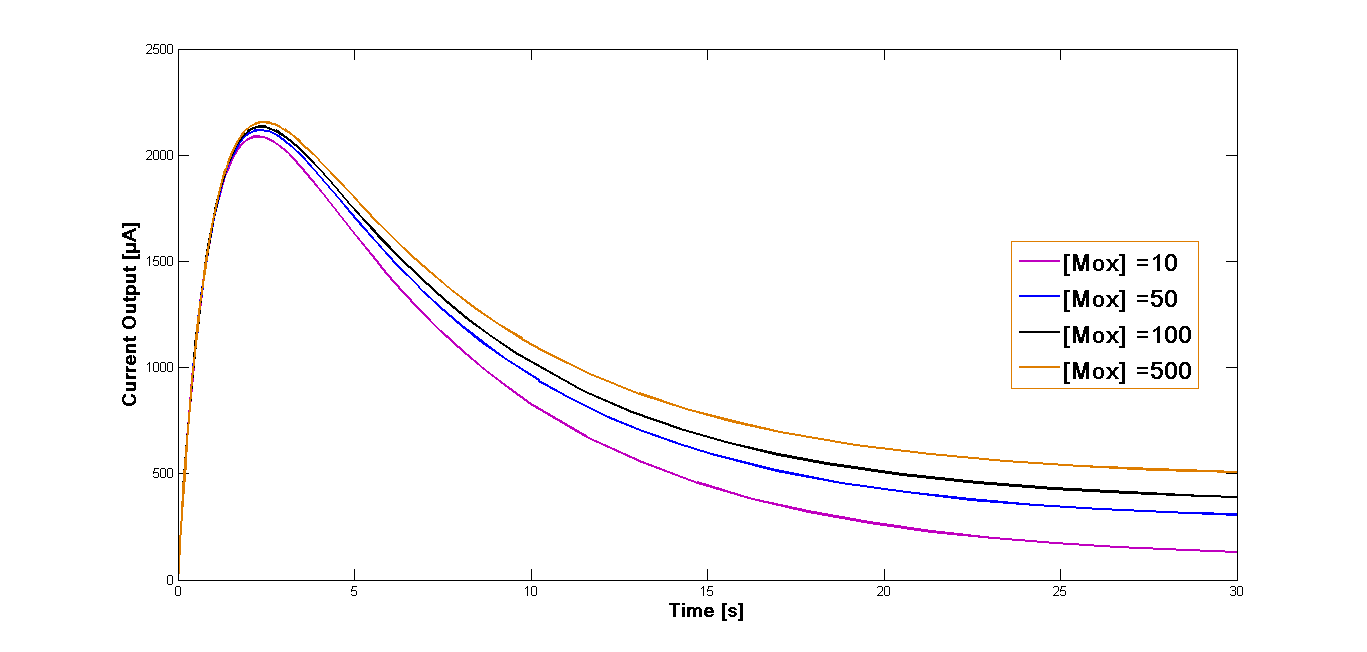
| + | Further simulation has been performed for four different start concentrations of the oxidized mediator in order to investigate how varying start concentration influence the current output. The concentrations were set at 10 µM, 50 µM, 100 µM and 500 µM. The time span was set at 30 sec. The curves of the current outputs are presented in the Figure 2. | ||
| + | <br><br> | ||
| + | [[File:Bielefeld-germany-model-oxid-diagramm-MB-02.png|600px|center|thumb|'''Figure 2''': Curves for the current output and fluxes of concentration of the intermediates. Simulation performed for the varying start concentration of the oxidized mediator '''MB''' [M<sub>ox</sub>] .]] | ||
| + | <br><br> | ||
| - | The | + | The MATLAB source code for both simulations can be obtained [https://static.igem.org/mediawiki/2013/2/27/Bielefeld-germany-model-oxid-matlab-code-1.m here] and [https://static.igem.org/mediawiki/2013/3/36/Bielefeld-germany-model-oxid-matlab-code-2.m here].<br> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
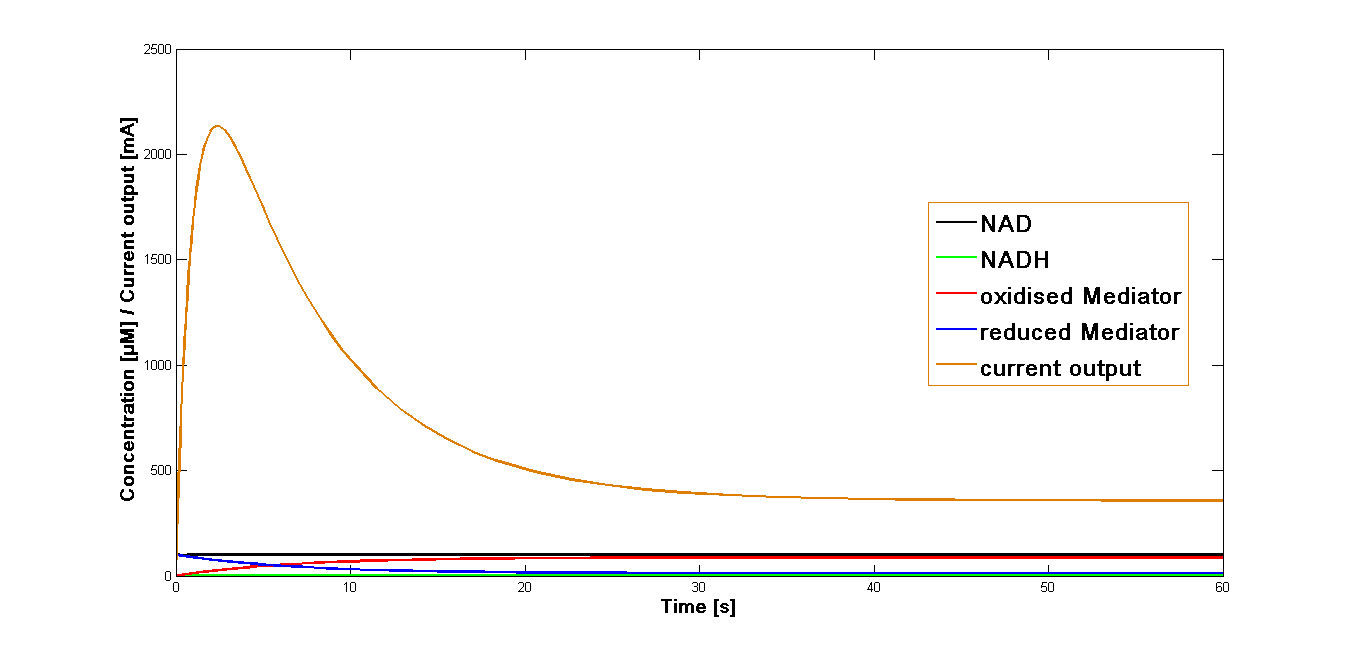
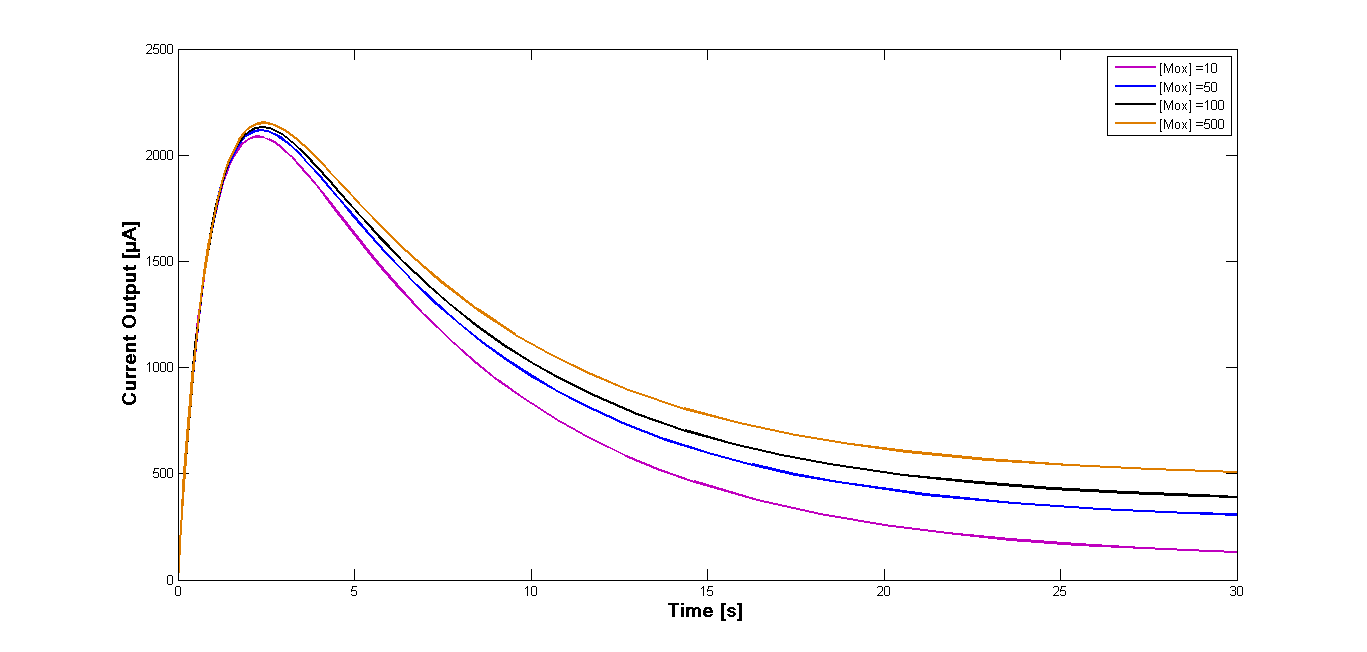
| + | Analogous simulations have been performed for the mediator '''NMP'''. As the reaction rate ''k<sub>3</sub>'' specific for this mediator could not be obtained, the value specific for the mediator '''MB''' was applied. The diagrams presenting the results of those simulations are shown in the Figure 3 and 4 respectively. | ||
| + | <br><br> | ||
| + | [[File:Bielefeld-germany-model-oxid-diagramm-NMP-03.png|600px|center|thumb|'''Figure 3''': Curves for the current output, fluxes of concentration of the intermediates. Simulation performed for the start concentration of both NAD<sup>+</sup> and oxidized mediator '''NMP''' at 100 µM.]] | ||
| + | <br><br> | ||
| + | [[File:Bielefeld-germany-model-oxid-diagramm-NMP-04.png|600px|center|thumb|'''Figure 4''': Curves for the current output and fluxes of concentration of the intermediates. Simulation performed for the varying start concentration of the oxidized mediator '''NMP''' [M<sub>ox</sub>] .]] | ||
| + | <br> | ||
| + | The according .m files with MATLAB source code can be downloaded from [https://static.igem.org/mediawiki/2013/b/be/Bielefeld-germany-model-oxid-matlab-code-3.m here] and [https://static.igem.org/mediawiki/2013/9/90/Bielefeld-germany-model-oxid-matlab-code-4.m here]. | ||
| + | </p> | ||
| + | ==Discussion== | ||
| + | <p align="justify"> | ||
| + | The curve for the current output simulated for the mediator '''MB''' has been presented in the Figure 1. It could be shown that the maximal output is reached rapidly after the start of the reaction system. Immediately after reaching the maximum, it decreases equally fast. At the 20 second mark it reaches a stable phase of about 20 per cent of the maximal current output.<br> | ||
| + | The comparison of different start concentrations of the oxidized mediator can be obtained from the Figure 2. The curve form is identical for all four concentrations and the reached current values differ only slightly for the different simulations. Also the maximum output is almost identical and is reached at the same time for all four approaches. | ||
| + | Interestingly the curves obtained from the simulation for the mediator '''NMP''' are very similar to those for the mediator '''MB'''. This points that the reaction rate ''k<sub>3</sub>'' for the oxidation of the mediator at the anode plays a predominant role in generating current.<br> | ||
| + | Obviously it is necessary to constantly provide the system with the redox molecules in order to sustain the current at the high level.This builds a strong case for the usse of endogenous mediators that are supplied and regenerated by the bacteria themselves. | ||
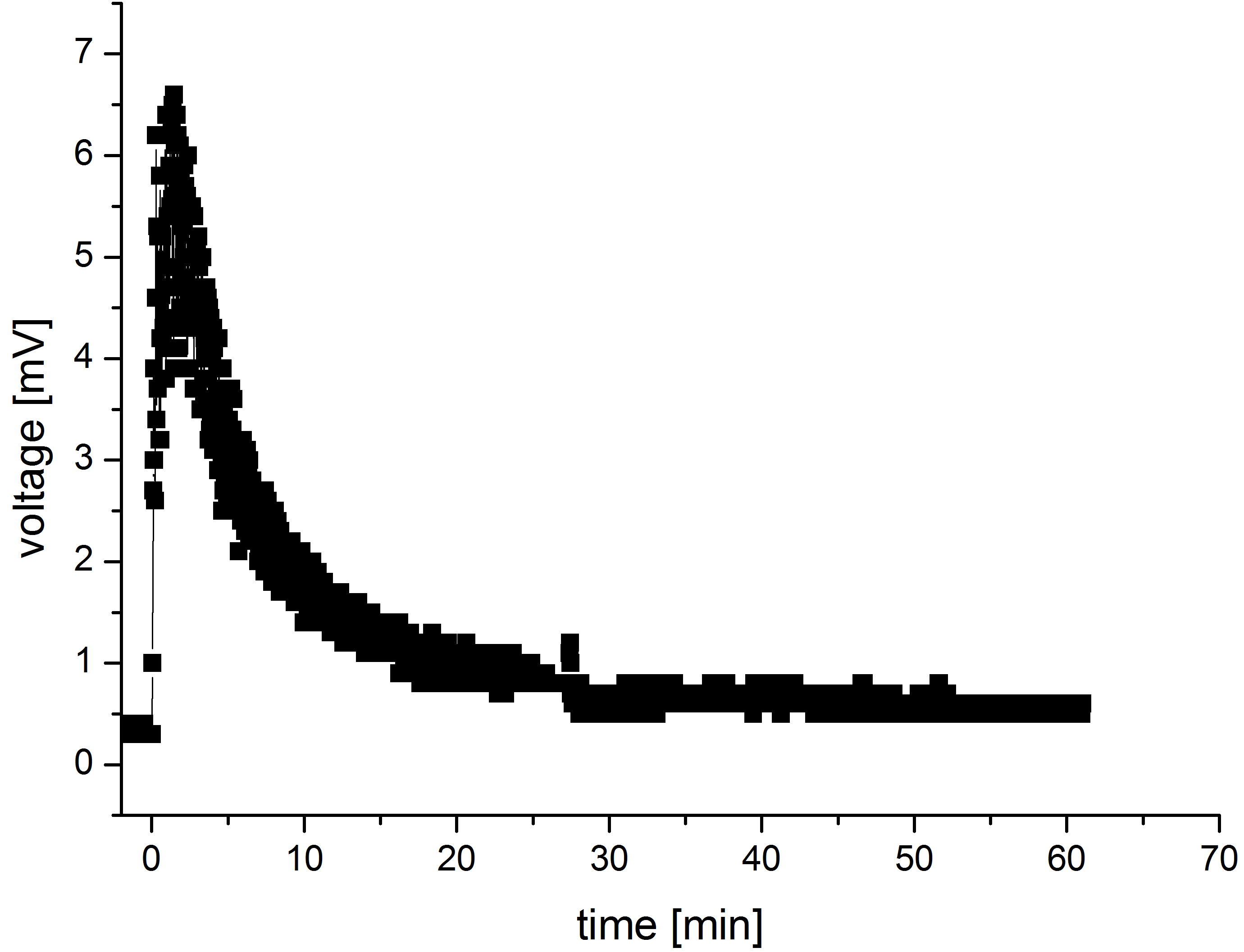
| + | Another interesting aspect emerges when comparing the modeled current output curves and those obtained with the values measured in MFC. The measurements were performed in a self-designed MFC where only M9 medium and no bacteria was present in the anode chamber.The resulting curve has been shown in the figure 5. The mediator was added to the anode chamber only once at the beginning of the measurements. | ||
| + | <br><br> | ||
| + | [[File:Bielefeld-germany-model-oxid-discus-01.jpg|450px|center|thumb|'''Figure 5''': .]] | ||
| + | <br> | ||
| + | The resulting curve form is very similar to the curves observed in the models. There are though significant differences in the dimensions of both current/voltage and time span in which the reactions take place. | ||
| + | Those differences might result from the fact that in our model we did not take the resistance into consideration. | ||
| + | </p> | ||
==References== | ==References== | ||
| - | |||
<br><br><br><br> | <br><br><br><br> | ||
</div> | </div> | ||
| - | |||
| - | |||
Latest revision as of 03:57, 29 October 2013
Modelling - Mediator Oxidation
Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator and
Mox the oxidized mediator.
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate of the mediator oxidation at the anode.
Simulation
The value k3 obtained as mentioned above was applied in the simulation of the current output in the final reaction of the three-reaction model. The start concentrations of the NAD+ and oxidized mediator were set as in the simulations preformed for the proceeding reactions at 100 µM. The simulation time span was set for 60 sec.
The resulting plot for the mediator MB is shown in the Figure 1, below:
Further simulation has been performed for four different start concentrations of the oxidized mediator in order to investigate how varying start concentration influence the current output. The concentrations were set at 10 µM, 50 µM, 100 µM and 500 µM. The time span was set at 30 sec. The curves of the current outputs are presented in the Figure 2.
The MATLAB source code for both simulations can be obtained here and here.
Analogous simulations have been performed for the mediator NMP. As the reaction rate k3 specific for this mediator could not be obtained, the value specific for the mediator MB was applied. The diagrams presenting the results of those simulations are shown in the Figure 3 and 4 respectively.
The according .m files with MATLAB source code can be downloaded from here and here.
Discussion
The curve for the current output simulated for the mediator MB has been presented in the Figure 1. It could be shown that the maximal output is reached rapidly after the start of the reaction system. Immediately after reaching the maximum, it decreases equally fast. At the 20 second mark it reaches a stable phase of about 20 per cent of the maximal current output.
The comparison of different start concentrations of the oxidized mediator can be obtained from the Figure 2. The curve form is identical for all four concentrations and the reached current values differ only slightly for the different simulations. Also the maximum output is almost identical and is reached at the same time for all four approaches.
Interestingly the curves obtained from the simulation for the mediator NMP are very similar to those for the mediator MB. This points that the reaction rate k3 for the oxidation of the mediator at the anode plays a predominant role in generating current.
Obviously it is necessary to constantly provide the system with the redox molecules in order to sustain the current at the high level.This builds a strong case for the usse of endogenous mediators that are supplied and regenerated by the bacteria themselves.
Another interesting aspect emerges when comparing the modeled current output curves and those obtained with the values measured in MFC. The measurements were performed in a self-designed MFC where only M9 medium and no bacteria was present in the anode chamber.The resulting curve has been shown in the figure 5. The mediator was added to the anode chamber only once at the beginning of the measurements.
The resulting curve form is very similar to the curves observed in the models. There are though significant differences in the dimensions of both current/voltage and time span in which the reactions take place.
Those differences might result from the fact that in our model we did not take the resistance into consideration.
References
 "
"