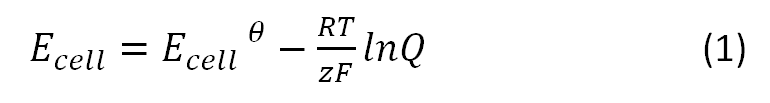Team:Bielefeld-Germany/Modelling/Optimal
From 2013.igem.org
Modelling - Optimal conditions
Overview
The Microbial Fuel Cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two parameters, namely the pH value and the temperature. All values were calculated based on the [http://en.wikipedia.org/wiki/Nernst_equation Nernst equation], which describes the relation between the electromotive force of the full cell and the temperature or the pH-value:
Optimal pH value
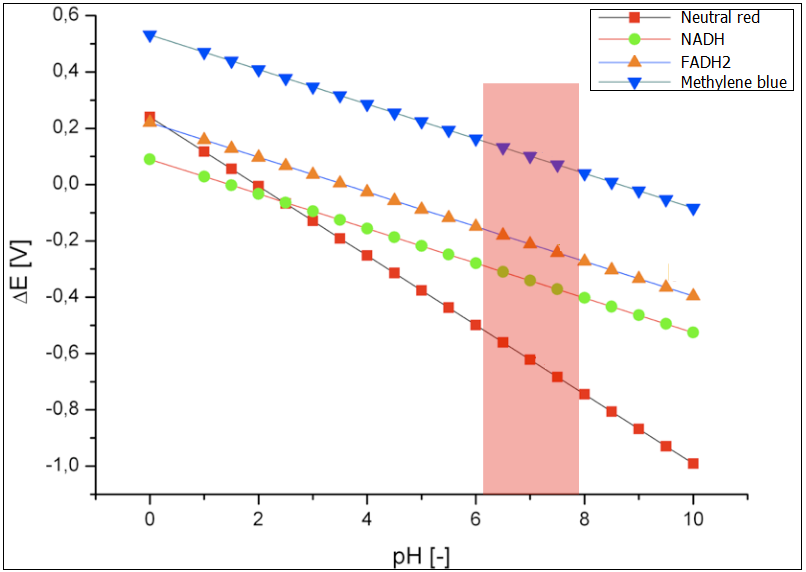
The following exogenous mediators were analyzed under varying pH-value: Neutral red, NADH, FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of E.coli have been marked in red. Methylene blue has been chosen as the proof of concept for the experimental examination of the fuel cell, after various initial tests. Hence, it has been in the focus of the modeling of the performance as well. The reduction potential for the optimal growth conditions for E.coli, is between ~0.13 and ~0.8 V. The reduction potential for neutral red lies in this pH interval is between -0.4 and -0.7. This value is too low and would not lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2.
Optimal temperature
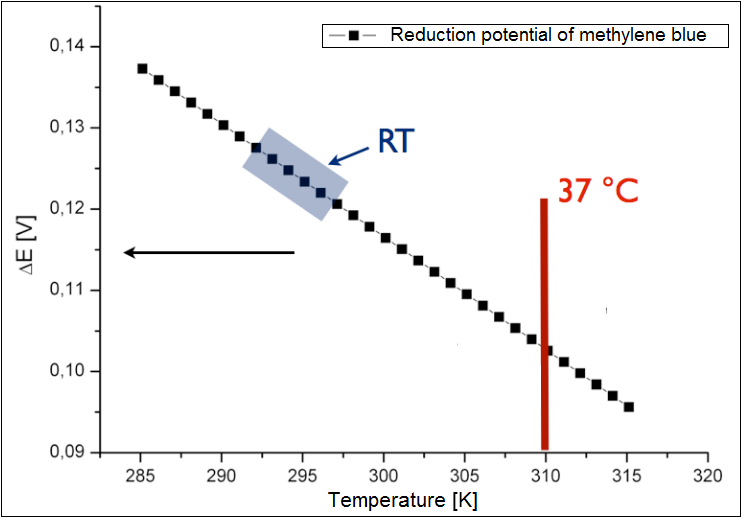
The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 2 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 .
Combined calculations for the proof system
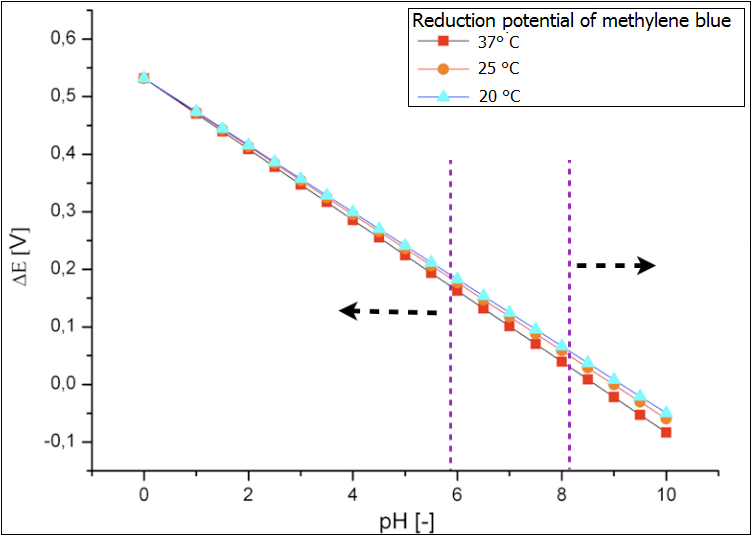
Ultimately the analysis of the influence of both temperature and pH value on the reduction potential of the proof system methylene blue has been performed. Its results are depicted in the figure 3. Again it could be shown that the temperature does not affect the reduction potential and therefore the effectiveness of the fuel cell. In contrast the pH-value in the chamber should be monitored and kept at the values that correspond to the optimal growth condition of the E.coli cells. The values below 6 would lead to too high reduction potential via the accumulation of the fermentation products under anaerob cultivation and decreased efficiency. Too basic conditions in the chamber (above 8) would lead to oxidation of the glucose and to so-called Blue-Bottle-Effect. The performance of the fuel cell would be also worse.
 "
"