Team:Bielefeld-Germany/Modelling/Oxidation
From 2013.igem.org
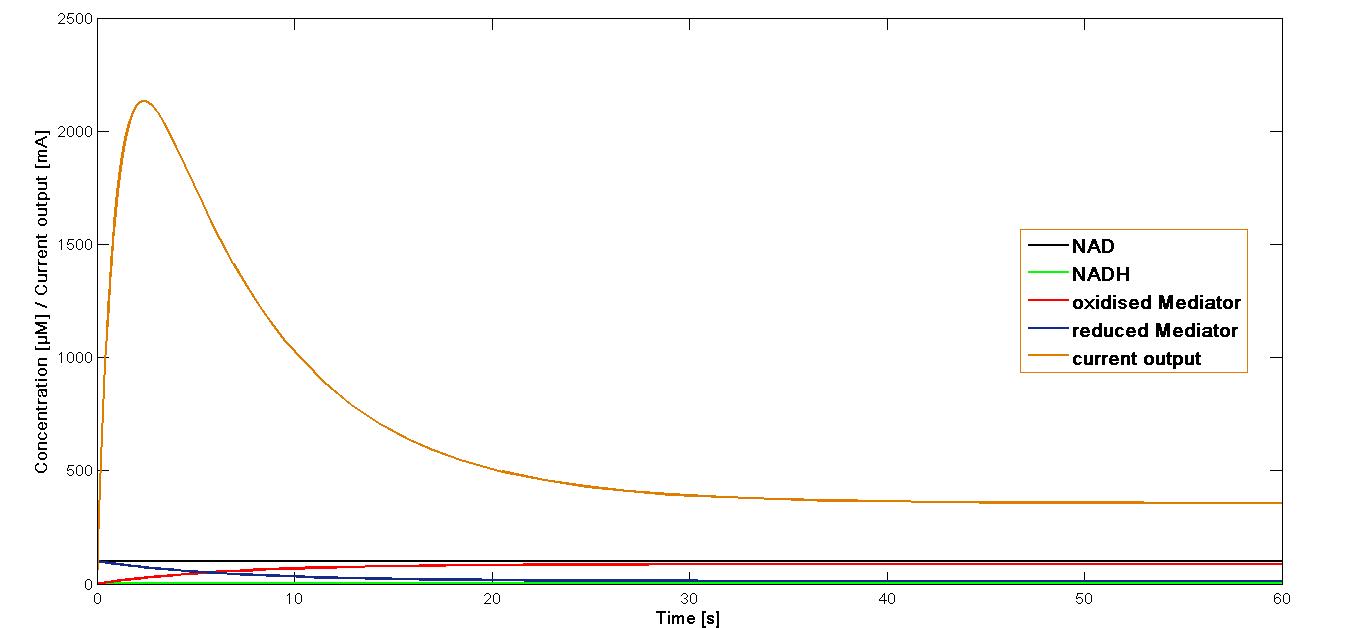
Modelling - Mediator Oxidation
Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator,
Mox the oxidized mediator and
k3 is the rate constant of the reaction
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate, mentioned above.
Simulation
References
 "
"