Team:ETH Zurich/Experiments 7
From 2013.igem.org
| Line 9: | Line 9: | ||
The set of hydrolases we use include the ''Citrobacter'' alkaline phospohatase, the ''Bacillus subtilis'' β-glucuronidase and the ''Escherichia coli'' acetyl esterase, β-N-acetylglucosaminidase and β-galactosidase. These enzymes have features that make them attractive as reporters. They are known to be relatively stable, exhibit activity under different conditions (e.g. pH and temperature), and catalyze the cleavage of various colorimetric and fluorescent substrates, which is ideal for visual screening. These hydrolases are all native to ''Escherichia coli'' and orthogonal to each other, which means that each substrate can only be processed by the corresponding specific enzyme. In order to prevent background expression of these native enzymes, we use a [https://2013.igem.org/Team:ETH_Zurich/Mutant triple knockout strain] that has three hydrolase genes knocked out: ''uidA'' (β-glucuronidase), ''aes'' (acetyl esterase) and ''nagZ'' (β-N-acetylglucosaminidase). </p> | The set of hydrolases we use include the ''Citrobacter'' alkaline phospohatase, the ''Bacillus subtilis'' β-glucuronidase and the ''Escherichia coli'' acetyl esterase, β-N-acetylglucosaminidase and β-galactosidase. These enzymes have features that make them attractive as reporters. They are known to be relatively stable, exhibit activity under different conditions (e.g. pH and temperature), and catalyze the cleavage of various colorimetric and fluorescent substrates, which is ideal for visual screening. These hydrolases are all native to ''Escherichia coli'' and orthogonal to each other, which means that each substrate can only be processed by the corresponding specific enzyme. In order to prevent background expression of these native enzymes, we use a [https://2013.igem.org/Team:ETH_Zurich/Mutant triple knockout strain] that has three hydrolase genes knocked out: ''uidA'' (β-glucuronidase), ''aes'' (acetyl esterase) and ''nagZ'' (β-N-acetylglucosaminidase). </p> | ||
| - | + | <p align="justify">Addition of a multi-substrate mix by the player leads to an enzyme-susbtrate reaction which specifically cleaves the chromogenic substrates, thereby producing a visible color output. Chromogenic substrates incorporate a chromophore whose absorbance properties change after the enzyme reaction, and the color signal produced then is directly related to the enzyme-catalyzed reaction ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 1]).</p> | |
| - | < | + | [[File:Rainbow hydrolases.JPG|470px|right|thumb|<b>Figure 1: Colorimetric output.</b> This image shows liquid cultures of our mutant ''Escherichia coli'' strain expressing the enzymes PhoA, NagZ, LacZ, Aes or GusA to convert specific chromogenic substrates into colored outputs.]] |
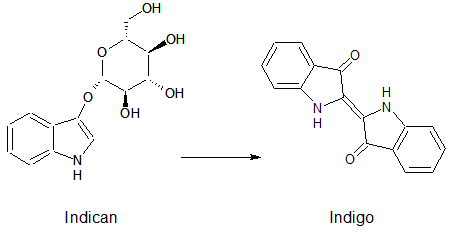
| - | Indican is a chromogenic glycoside hydrolase substrate which belongs to a family of natural glycosides found in plants. Cleavage of the glycosidic bond forms an unstable hydroxyindole intermediate, which dimerizes by oxidation to form indigo as a blue precipitate:</p> | + | |
| + | <p align ="justify">Indican is a chromogenic glycoside hydrolase substrate which belongs to a family of natural glycosides found in plants. Cleavage of the glycosidic bond forms an unstable hydroxyindole intermediate, which dimerizes by oxidation to form indigo as a blue precipitate:</p> | ||
[[File:IndicanIndigo.png|275px|center|]] | [[File:IndicanIndigo.png|275px|center|]] | ||
<p align="justify">Numerous enzyme substrates have been designed following this natural product example, giving rise to many colored phenols that are used to detect enzyme activities.<br><br> | <p align="justify">Numerous enzyme substrates have been designed following this natural product example, giving rise to many colored phenols that are used to detect enzyme activities.<br><br> | ||
| - | Some of the hydrolases used in the Colisweeper reporter system can catalyze hydrolysis of various substrates, with different chromophores that give rise to a wide range of colors. This variety of substrates and colored outputs, additionally to the orthogonality of the enzyme-substrate reactions, enables usage of multiple hydrolases in reporter gene assays, which are becoming more important in real-time detection of cellular events associated with gene expression, regulation and signal transduction ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference | + | Some of the hydrolases used in the Colisweeper reporter system can catalyze hydrolysis of various substrates, with different chromophores that give rise to a wide range of colors. This variety of substrates and colored outputs, additionally to the orthogonality of the enzyme-substrate reactions, enables usage of multiple hydrolases in reporter gene assays, which are becoming more important in real-time detection of cellular events associated with gene expression, regulation and signal transduction ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 2]). |
<br><br> | <br><br> | ||
Revision as of 17:10, 24 October 2013
Reporter system
Colisweeper depends on the processing of molecular signals and generation of visible and distinguishable color outputs. By using the wild type PLuxR promoter and mutant promoters with varying AHL/LuxR affinities, gene expression can be induced for different concentrations of AHL. We coupled this system with reporter enzymes, called hydrolases, which catalyze hydrolysis of their colorless substrate into a colored product. Therefore, the player only triggers the colored response upon addition of a substrate.
The set of hydrolases we use include the Citrobacter alkaline phospohatase, the Bacillus subtilis β-glucuronidase and the Escherichia coli acetyl esterase, β-N-acetylglucosaminidase and β-galactosidase. These enzymes have features that make them attractive as reporters. They are known to be relatively stable, exhibit activity under different conditions (e.g. pH and temperature), and catalyze the cleavage of various colorimetric and fluorescent substrates, which is ideal for visual screening. These hydrolases are all native to Escherichia coli and orthogonal to each other, which means that each substrate can only be processed by the corresponding specific enzyme. In order to prevent background expression of these native enzymes, we use a triple knockout strain that has three hydrolase genes knocked out: uidA (β-glucuronidase), aes (acetyl esterase) and nagZ (β-N-acetylglucosaminidase).
Addition of a multi-substrate mix by the player leads to an enzyme-susbtrate reaction which specifically cleaves the chromogenic substrates, thereby producing a visible color output. Chromogenic substrates incorporate a chromophore whose absorbance properties change after the enzyme reaction, and the color signal produced then is directly related to the enzyme-catalyzed reaction (1).
Indican is a chromogenic glycoside hydrolase substrate which belongs to a family of natural glycosides found in plants. Cleavage of the glycosidic bond forms an unstable hydroxyindole intermediate, which dimerizes by oxidation to form indigo as a blue precipitate:
Numerous enzyme substrates have been designed following this natural product example, giving rise to many colored phenols that are used to detect enzyme activities.
Some of the hydrolases used in the Colisweeper reporter system can catalyze hydrolysis of various substrates, with different chromophores that give rise to a wide range of colors. This variety of substrates and colored outputs, additionally to the orthogonality of the enzyme-substrate reactions, enables usage of multiple hydrolases in reporter gene assays, which are becoming more important in real-time detection of cellular events associated with gene expression, regulation and signal transduction (2).
For most parts encoding the hydrolases that were sent to the registry by our team, we have conducted fluorometric assays to study and compare their kinetics. The results can be seen here, as well as in the experience section of the parts' registry sites, and the methods used are available here.
Click on the hydrolases to learn more about them
References
(1) Reymond JL, Fluxà VS, Maillard N, Chem. Commun., 1, 34 – 46 (2009).
(2) Jiang T, Xing B, Rao J, Biotechnology and Genetic Engineering Reviews, 25, 41-76 (2008).
(3) Kanaya S, Koyanagi T, Kanaya E, Biochem. J., 332, 75-80 (1998).
(4) Peist R, Koch A, Bolek P, Sewitz S, Kolbus T, Boos J, J. Bacteriol., 179, 7679-7686 (1997).
(5) Blattner FR, Plunkett III G, Bloch CA, Perna NT, Burna V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y, Science, 277, 1453-1461 (1997).
(6) Haruki M, Oohashi Y, Mizuguchi S, Matsuo Y, Morikawa M, Kanaya S, FEBS Lett., 454, 262-266 (1999).
(7) Farias T, Mandrich L, Rossi M, Manco G, Protein Pept. Lett., 14, 165-169 (2007).
(8) Kobayashi R, Hirano N, Kanaya S, Haruki M, Biosci. Biotechnol. Biochem., 76 (11), 2082-2088 (2012).
(9) Kim EE, Wyckoff HW, J. Mol. Biol., 218, 449-464 (1991).
(10) Yang K, Metcalf WW, PNAS, 101, 7919-7924 (2004).
(11) Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW, Biochemistry, 40, 14781-14794 (2001).
(12)Russell WM, Klaenhammer TR, Appl. Environ. Microbiol., 67, 1253-1261 (2000).
(13)Beaud D, Tailliez P, Anba-Mondoloni J, Microbiology, 171, 2323-2330 (2005).
(14)Jefferson RA, Burgess SM, Hirsh D, Procl. Natl. Acad. Sci. USA, 83, 8447-8451 (1986).
(15)Tryland I, Fiksdal L, Appl. Environ. Microbiol., 64, 1018-1023 (1997).
(16) Cheng Q, Li H, Merdek K, Park JT, J. Bacteriol., 182, 4836-4840 (2000).
(17) Yem DW, Wu HC, J. Bacteriol., 125, 324-331 (1976).
(18) Vötsch W, Templin MF, J. Biol. Chem., 275, 39032-39038 (2000).
 "
"