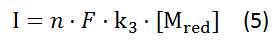Team:Bielefeld-Germany/Modelling/Oxidation
From 2013.igem.org
(Difference between revisions)
| Line 49: | Line 49: | ||
<h1>Modelling - Mediator Oxidation</h1> | <h1>Modelling - Mediator Oxidation</h1> | ||
| - | |||
<div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:10px; clear:both;"> | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:10px; clear:both;"> | ||
| Line 66: | Line 65: | ||
</div> | </div> | ||
</html> | </html> | ||
| - | |||
==Mediator Oxidation== | ==Mediator Oxidation== | ||
Revision as of 21:27, 27 October 2013
Modelling - Mediator Oxidation
Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator,
Mox the oxidized mediator and
k3 is the rate constant of the reaction
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate, mentioned above.
References
 "
"