Team:Bielefeld-Germany/Modelling/Oxidation
From 2013.igem.org
m |
m |
||
| Line 71: | Line 71: | ||
[[File:Bielefeld-germany-model-oxid-reaction-1.PNG|300px|center]] | [[File:Bielefeld-germany-model-oxid-reaction-1.PNG|300px|center]] | ||
| - | |||
<br> | <br> | ||
| - | |||
| - | |||
| + | ,where M<sub>red</sub> is the reduced mediator and <br> | ||
| + | M<sub>ox</sub> the oxidized mediator. | ||
| + | <br> | ||
Then the current output can be calculated based on formula according to the Faraday's law:<br> | Then the current output can be calculated based on formula according to the Faraday's law:<br> | ||
| Line 92: | Line 92: | ||
==Simulation== | ==Simulation== | ||
<p align="justify"> | <p align="justify"> | ||
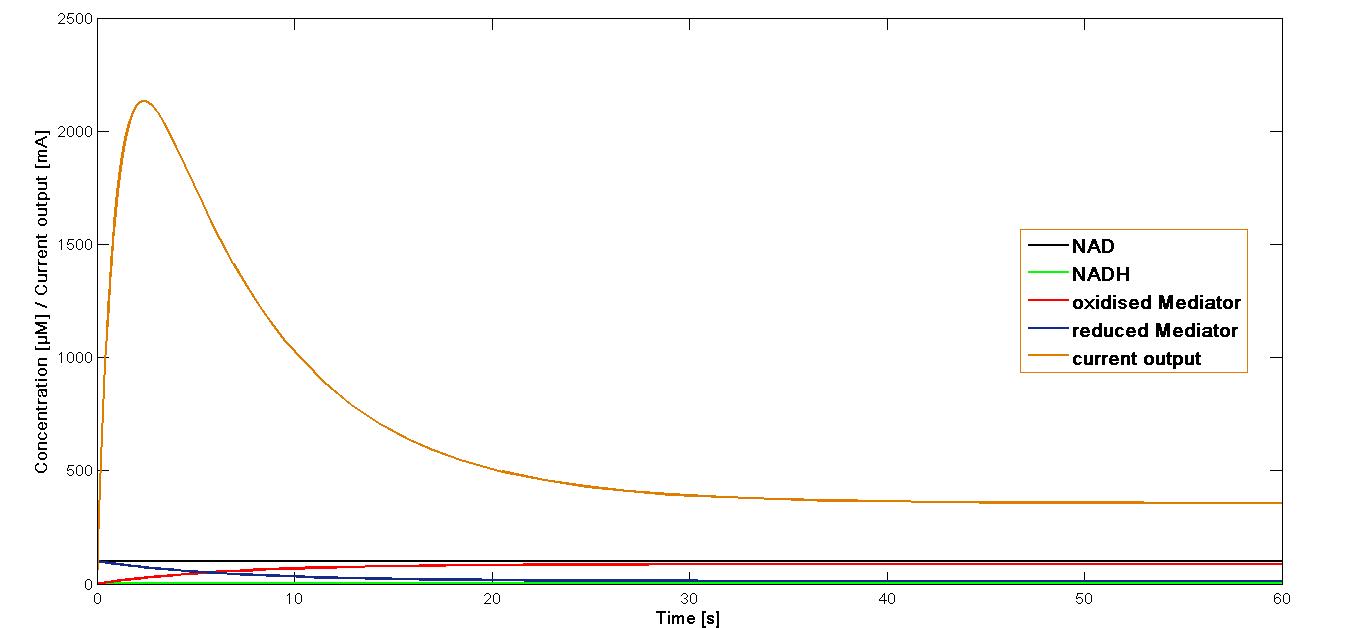
| - | The | + | The value ''k<sub>3</sub>'' obtained as mentioned above was applied in the simulation of the current output in the final reaction of the three-reaction model. The start concentrations of the NAD<sup>+</sup> and oxidized mediator were set as in the simulations preformed for the proceeding reactions at 100 µM. The simulation time span was set for 60 sec. |
The resulting plot for the mediator '''MB''' is shown in the figure1 below: | The resulting plot for the mediator '''MB''' is shown in the figure1 below: | ||
Revision as of 00:57, 29 October 2013
Modelling - Mediator Oxidation
Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator and
Mox the oxidized mediator.
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate, mentioned above.
Simulation
The value k3 obtained as mentioned above was applied in the simulation of the current output in the final reaction of the three-reaction model. The start concentrations of the NAD+ and oxidized mediator were set as in the simulations preformed for the proceeding reactions at 100 µM. The simulation time span was set for 60 sec. The resulting plot for the mediator MB is shown in the figure1 below:
Further simulation has been performed for four different start concentrations of the oxidised mediator in order to investigate how varying start concentration influence the current output. The concentrations were set at 10 µM, 50 µM, 100 µM and 500 µM. The time span was set at 30 sec.
The MATLAB source code for both simulations can be obtained here and here.
Analogous simulations have been performed for the mediator NMP. As the reaction rate k3 specific of the could not be accessed, the value specific for the mediator MB was applied.
The according .m files with MATLAB source code can be downloaded from here and here.
References
 "
"