Team:ETH Zurich/Experiments 6
From 2013.igem.org
| (87 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{:Team:ETH_Zurich/Templates/stylesheet}} | {{:Team:ETH_Zurich/Templates/stylesheet}} | ||
| - | <h1>GFP | + | <h1>GFP diffusion tests with sender cells and wild-type P<sub>LuxR</sub> receiver constructs</h1> |
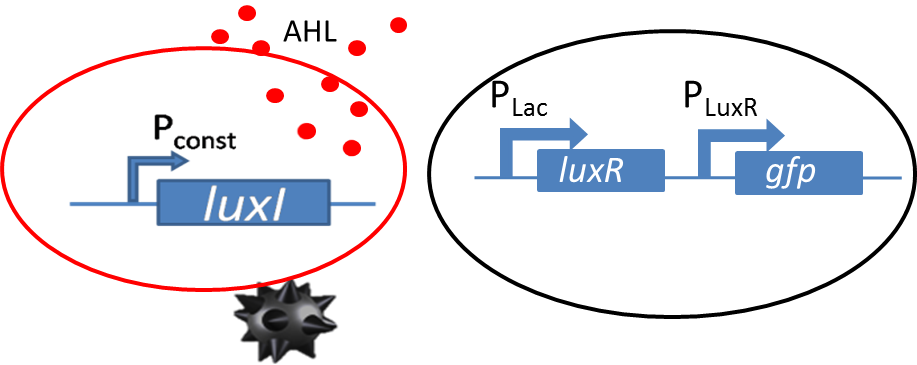
| - | [[File: | + | [[File:Mine_and_only_mine.png|left|400px|thumb|<b>Figure 1: </b> Sender receiver biological circuit with GFP as reporter.]] |
| - | <p align= "justify">Diffusion experiments were performed to study | + | <p align="justify">Diffusion experiments were performed to study AHL diffusion from the sender colonies to the receiver colonies. The sender receiver circuit is shown in the figure to the left. The sender colonies consist of the LuxI ([http://parts.igem.org/Part:BBa_K805016 BBa_K805016]) producing AHL under a constitutive promoter. The luxI produces AHL that diffuses through the agar. The diffused AHL reaches the non-mine and is processed via promoter part [http://parts.igem.org/Part:BBa_J09855 BBa_J09855] cloned with GFP was used as our receiver. The presence of GFP in the receiver colonies is then co-related to the amount of AHL processed by the receiver colonies.</p> |
| - | The | + | <br><br> |
| - | |||
<br clear="all"/> | <br clear="all"/> | ||
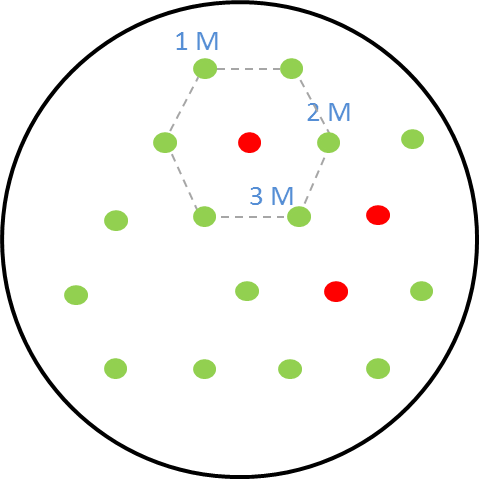
| + | [[File:i love you.png|right|270px|thumb|<b>Figure 2: Schematic diagram of placement of sender receiver colonies</b> in the hexagonal grid with one, two and three mines.]] | ||
| - | |||
| - | |||
| - | <p align= "justify"> The | + | <p align="justify">The picture on the right shows the schematic diagram of the experimental set-up for the GFP as reporter in the receiver cells. The colonies are placed in a hexagonal grid pattern. The circles represent colonies on the agar plate. The green circles are the non-mine receiver cells with <b>GFP as the reporter</b>. The circles that are colored red are the mine colonies that secrete the signalling molecule <b>AHL.</b> As our mine grid design is a honeycomb pattern, we play the game with one, two and three mines. This explanation is given [https://2013.igem.org/Team:ETH_Zurich/Play here.] The numbers <b>1, 2 and 3</b> in the diagram represent the receiver colonies that are surrounded <b> by one, two and three mines</b> respectively. The same set-up was used for the experiments and the florescence images were taken of the receiver colonies at various time points.</p> |
| - | + | <br><br><br> | |
| - | The | + | <br clear="all"/> |
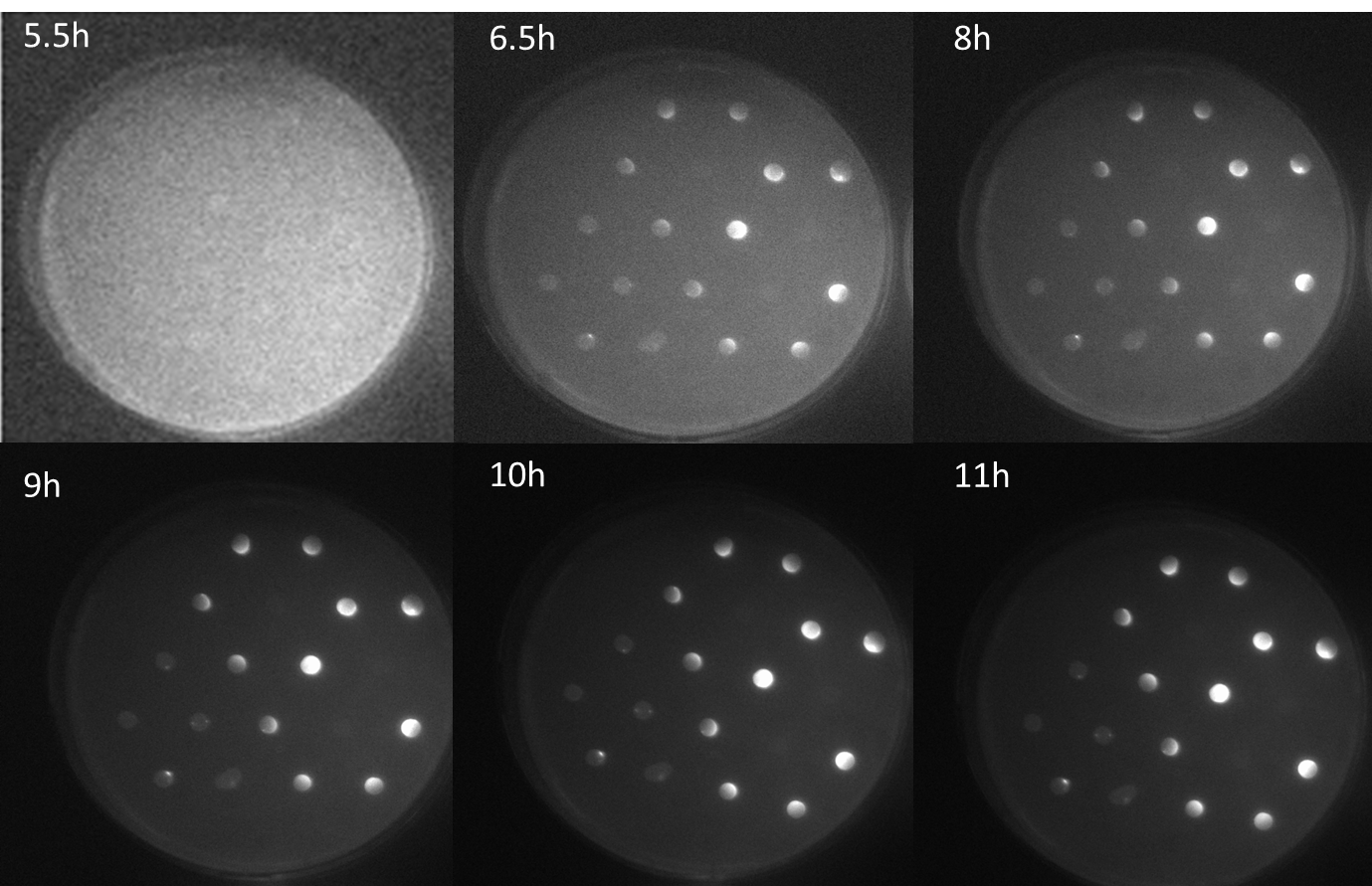
| - | < | + | <p align= "justify">The figure shows a series of scanned fluorescence images of the agar plate with the sender-receiver colonies plated in the same manner as in the schematic diagram. The images were taken every half hour since the first fluorescence was observed. This was observed at 5.5 hours after incubation at 37 °C. At nearly 6.6 h, difference in the GFP levels can be observed in colonies The fluorescence increases with time at 8 h and 9 h. At 11 h, maximum fluorescence is seen after which the GFP fluorescence saturation levels were reached. The important feature from these images is that, different levels of fluorescence is observed in the colonies depending on the number of mines colonies around the GFP receiver colonies. <b>The highest fluorescence is seen in the colony surrounded by three mines. The next highest fluorescence in colonies surrounded with two mines and the least in colonies adjacent to one mine. Another striking feature is the gradient of fluorescence within one colony itself that is dependent on the direction of diffused AHL within this receiver colony. This experiment thus stands as a proof-of-concept of our bio-game as we are clearly able to differentiate colonies adjacent 1, 2 and 3 mine colonies</b>.</p> |
| - | + | ||
| - | |||
| + | [[File:a miracle.png|center|750px|thumb|<b>Figure 3: Scanned images of GFP fluorescence over time on an agar plate with sender colonies and GFP receiver colonies </b> in a hexagonal grid with one, two and three mines.]] | ||
| + | <br><br> | ||
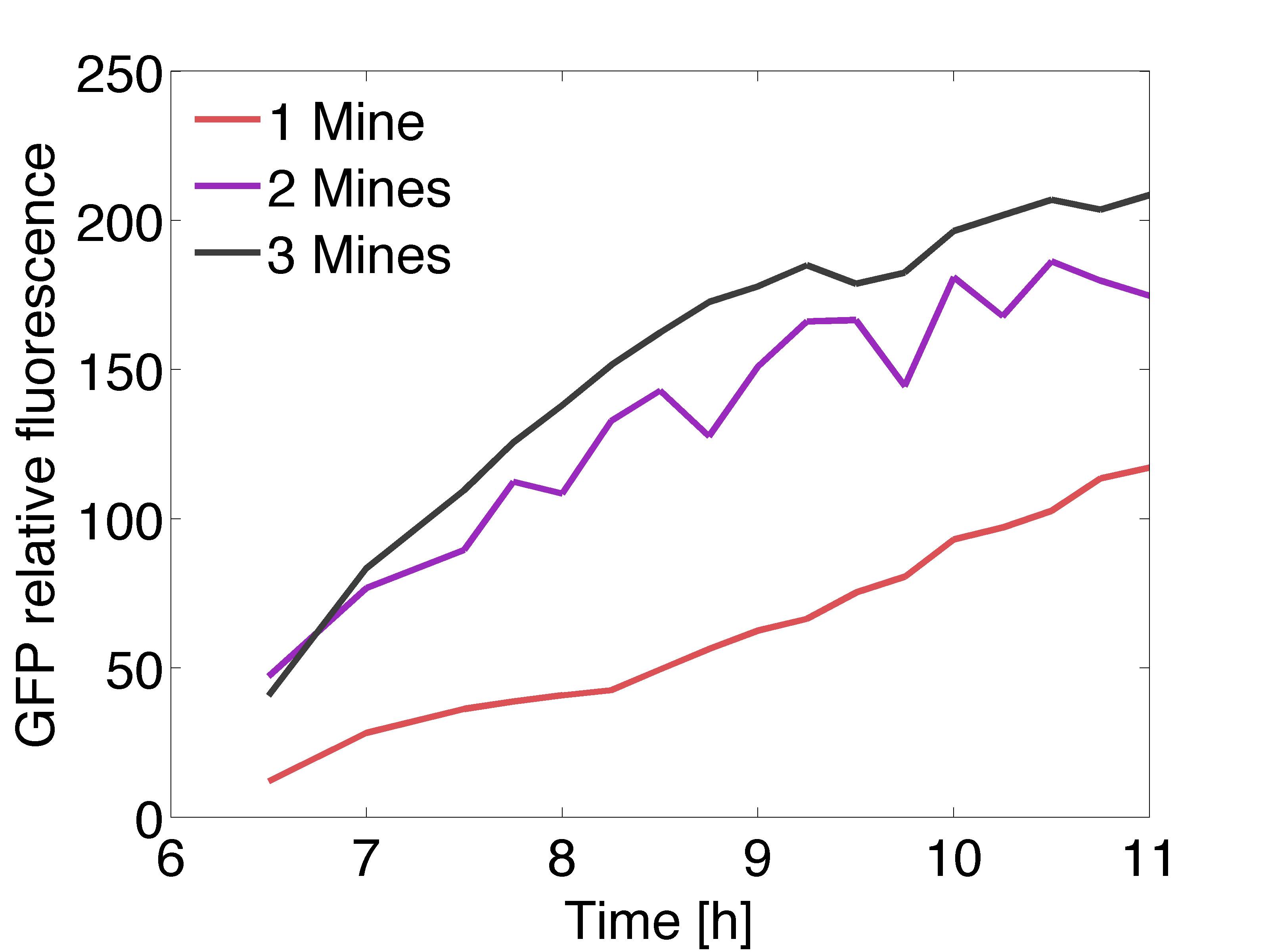
| + | [[File:Gfpexp_pop.png|right|450px|thumb|<b>Figure 4: Quantitative data of the relative GFP fluorescence in a receiver colony </b> surrounded by one (red line), two (purple line) and three (black line) mine colonies over time.]] | ||
| - | + | <p align= "justify">The graph on the right shows the relative intensity of the GFP signal in the receiver colonies with one, two and three adjacent mine colonies at different timepoints. All scanned images of the plate similar to the one depicted above were analyzed using the image processing program ImageJ. The GFP fluorescence reaches saturation after 11 hours of incubation at 37℃ . Due to the presence of more mine colonies, more AHL molecules are processed by the non-mine and higher fluorescence can be observed. Three adjacent mines lead to the highest value of fluorescence compared to two or one adjacent mine colonies. This data is qualitatively similar to the model, but GFP expression is quantitatively different in comparison to the model. | |
| + | </p> | ||
| + | <br clear="all"/> | ||
| + | <h1>GFP diffusion tests with sender cells and mutated P<sub>luxR</sub> promoter receiver constructs </h1> | ||
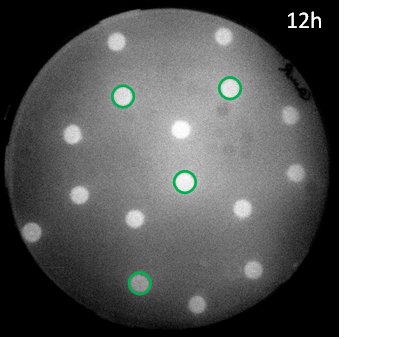
| + | [[File:G1grid12h.png|right|250px|thumb|<b>Figure 5: Scanned image of GFP fluorescence in a hexagonal grid with sender cells </b> in green and mutant pLux GFP receiver cells]] | ||
| + | |||
| + | <p align= "justify"> Also the mutant promoter [http://parts.igem.org/Part:BBa_K1216007 BBa_K1216007] we obtained through [https://2013.igem.org/Team:ETH_Zurich/Experiments_5 site-saturation mutagenesis] was tested in the hexagonal grid experiment with LuxI sender cells. The sensitivity for AHL (EC<sub>50</sub>=12'555 nM) was very low and only in the case where three mines surround a receiver colony some fluorescence could be observed. Still the results are consistent with the model predictions and led to the rational design of additional promoter mutants, where either one of the two point mutions was changed back with the goal to recover some of the sensitivity of the wild type. | ||
| + | <br><br> | ||
| + | [[File:Wt g1 comparison.png|left|400px|thumb|<b>Figure 6: </b>Sequence of the wild type pLuxR promoter and the tested variant.]] | ||
| + | <br clear="all"/> | ||
| + | |||
| + | </p> | ||
<br clear="all"/> | <br clear="all"/> | ||
{{:Team:ETH_Zurich/templates/footer}} | {{:Team:ETH_Zurich/templates/footer}} | ||
Latest revision as of 01:54, 29 October 2013
GFP diffusion tests with sender cells and wild-type PLuxR receiver constructs
Diffusion experiments were performed to study AHL diffusion from the sender colonies to the receiver colonies. The sender receiver circuit is shown in the figure to the left. The sender colonies consist of the LuxI ([http://parts.igem.org/Part:BBa_K805016 BBa_K805016]) producing AHL under a constitutive promoter. The luxI produces AHL that diffuses through the agar. The diffused AHL reaches the non-mine and is processed via promoter part [http://parts.igem.org/Part:BBa_J09855 BBa_J09855] cloned with GFP was used as our receiver. The presence of GFP in the receiver colonies is then co-related to the amount of AHL processed by the receiver colonies.
The picture on the right shows the schematic diagram of the experimental set-up for the GFP as reporter in the receiver cells. The colonies are placed in a hexagonal grid pattern. The circles represent colonies on the agar plate. The green circles are the non-mine receiver cells with GFP as the reporter. The circles that are colored red are the mine colonies that secrete the signalling molecule AHL. As our mine grid design is a honeycomb pattern, we play the game with one, two and three mines. This explanation is given here. The numbers 1, 2 and 3 in the diagram represent the receiver colonies that are surrounded by one, two and three mines respectively. The same set-up was used for the experiments and the florescence images were taken of the receiver colonies at various time points.
The figure shows a series of scanned fluorescence images of the agar plate with the sender-receiver colonies plated in the same manner as in the schematic diagram. The images were taken every half hour since the first fluorescence was observed. This was observed at 5.5 hours after incubation at 37 °C. At nearly 6.6 h, difference in the GFP levels can be observed in colonies The fluorescence increases with time at 8 h and 9 h. At 11 h, maximum fluorescence is seen after which the GFP fluorescence saturation levels were reached. The important feature from these images is that, different levels of fluorescence is observed in the colonies depending on the number of mines colonies around the GFP receiver colonies. The highest fluorescence is seen in the colony surrounded by three mines. The next highest fluorescence in colonies surrounded with two mines and the least in colonies adjacent to one mine. Another striking feature is the gradient of fluorescence within one colony itself that is dependent on the direction of diffused AHL within this receiver colony. This experiment thus stands as a proof-of-concept of our bio-game as we are clearly able to differentiate colonies adjacent 1, 2 and 3 mine colonies.
The graph on the right shows the relative intensity of the GFP signal in the receiver colonies with one, two and three adjacent mine colonies at different timepoints. All scanned images of the plate similar to the one depicted above were analyzed using the image processing program ImageJ. The GFP fluorescence reaches saturation after 11 hours of incubation at 37℃ . Due to the presence of more mine colonies, more AHL molecules are processed by the non-mine and higher fluorescence can be observed. Three adjacent mines lead to the highest value of fluorescence compared to two or one adjacent mine colonies. This data is qualitatively similar to the model, but GFP expression is quantitatively different in comparison to the model.
GFP diffusion tests with sender cells and mutated PluxR promoter receiver constructs
Also the mutant promoter [http://parts.igem.org/Part:BBa_K1216007 BBa_K1216007] we obtained through site-saturation mutagenesis was tested in the hexagonal grid experiment with LuxI sender cells. The sensitivity for AHL (EC50=12'555 nM) was very low and only in the case where three mines surround a receiver colony some fluorescence could be observed. Still the results are consistent with the model predictions and led to the rational design of additional promoter mutants, where either one of the two point mutions was changed back with the goal to recover some of the sensitivity of the wild type.
 "
"