Team:Colombia Uniandes/NickoProject
From 2013.igem.org
Trafalmejo (Talk | contribs) |
|||
| (83 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Http://2013.igem.org/Team:Colombia Uniandes/Bootstrap}} | {{Http://2013.igem.org/Team:Colombia Uniandes/Bootstrap}} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
| - | </div> | + | <div class="container-fluid"> |
| - | </div> | + | <div class="row-fluid"> |
| - | + | <div class="span3"> | |
| + | <img src="https://static.igem.org/mediawiki/2013/8/89/Nicko.jpg" width="190" height="190"> | ||
| + | </div> | ||
| + | <div class="span9"> | ||
| + | <br> | ||
| + | <div class="accordion" id="accordion2"> | ||
| + | <div class="accordion-group"> | ||
| + | <div class="accordion-heading"> | ||
| + | <a class="accordion-toggle" data-toggle="collapse" data-parent="#accordion2" href="#collapseOne"> | ||
| + | Context | ||
| + | </a> | ||
| + | </div> | ||
| + | <div id="collapseOne" class="accordion-body collapse"> | ||
| + | <div class="accordion-inner"> | ||
| + | <h3 id="Nickel">Our Nickel Absorption System!</h3> | ||
</html> | </html> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<p align="justify"> | <p align="justify"> | ||
| - | Nickel is a silvery-white, hard, malleable, and ductile metal. Now days the major use of nickel is in the preparation of alloys which are characterized by their strength, ductility, and resistance to corrosion and heat, such as stainless steel. It is also used for rechargeable batteries, catalysts and other chemicals among others. Most nickel on Earth is inaccessible; this is why the metal is mined in Russia, Australia, Cuba, Canada, Colombia, Venezuela and South Africa. | + | Nickel is a silvery-white, hard, malleable, and ductile metal. Now days the major use of nickel is in the preparation of alloys which are characterized by their strength, ductility, and resistance to corrosion and heat, such as stainless steel. It is also used for rechargeable batteries, catalysts and other chemicals among others. Most nickel on Earth is inaccessible; this is why the metal is mined in Russia, Australia, Cuba, Canada, Colombia, Venezuela and South Africa<sup>1</sup>. |
</p> | </p> | ||
<p align="justify"> | <p align="justify"> | ||
| - | As a result of this activity all kinds of water bodies are being contaminated with metals of this nature, Colombia, is one of the many affected. In small quantities nickel is essential, but when the uptake is too high it can be a dangerous to human health bringing many types of cancer, birth defects, abortions, asthma, allergic reactions such as skin rashes or “nickel-itch”, and many other conditions as its consequences. | + | As a result of this activity all kinds of water bodies are being contaminated with metals of this nature, Colombia, is one of the many affected. In small quantities nickel is essential, but when the uptake is too high it can be a dangerous to human health<sup>2,3</sup> bringing many types of cancer, birth defects, abortions, asthma, allergic reactions such as skin rashes or “nickel-itch”, and many other conditions as its consequences. |
</p> | </p> | ||
<p align="justify"> | <p align="justify"> | ||
| - | Responding to these concerns, we have designed a bacterium which would be capable of absorbing the nickel in its interior using the internal regulating system of nickel belonging to the ''Cupriavidus metallidurans'', to later be removed magnetically, using the property from ''Magnetospirillum magneticum'' AMB-1 which will be used as our final chassis. | + | Responding to these concerns, we have designed a bacterium which would be capable of absorbing the nickel in its interior using the internal regulating system of nickel belonging to the ''Cupriavidus metallidurans'' (called ''Ralstonia metallidurans'' before), to later be removed magnetically, using the property from ''Magnetospirillum magneticum'' AMB-1 which will be used as our final chassis. |
</p> | </p> | ||
| + | <html> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <div class="accordion-group"> | ||
| + | <div class="accordion-heading"> | ||
| + | <a class="accordion-toggle" data-toggle="collapse" data-parent="#accordion2" href="#collapseTwo"> | ||
| + | Nickel Removal System | ||
| + | </a> | ||
| + | </div> | ||
| + | <div id="collapseTwo" class="accordion-body collapse"> | ||
| + | <div class="accordion-inner"> | ||
| + | <h3 id="parts">Design: Project Parts!</h3> | ||
| - | |||
| - | |||
<h4 id="nickelRemoval">Nickel Removal System</h4> | <h4 id="nickelRemoval">Nickel Removal System</h4> | ||
| - | |||
<h5 id="nickelConstruct">Our construct</h5> | <h5 id="nickelConstruct">Our construct</h5> | ||
| + | </html> | ||
<p align="justify"> | <p align="justify"> | ||
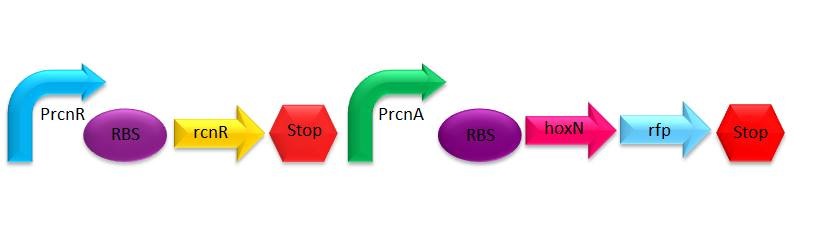
Using the nickel homeostatic system of ''E. coli'' and ''Cupriavidus metallidurans'', we will be able to create a system capable to detect and absorb nickel, to later be removed magnetically, using the magnetotactic property from ''Magnetospirillum magneticum'' AMB-1 which will be used as our final chassis. | Using the nickel homeostatic system of ''E. coli'' and ''Cupriavidus metallidurans'', we will be able to create a system capable to detect and absorb nickel, to later be removed magnetically, using the magnetotactic property from ''Magnetospirillum magneticum'' AMB-1 which will be used as our final chassis. | ||
</p> | </p> | ||
| - | + | [[File:Img.jpg | thumb | center | upright=3.0 | Nickel removal system's construct]] | |
| - | [[File:Img.jpg | + | <html> |
<h5 id="nickelExplanation">Explanation?</h5> | <h5 id="nickelExplanation">Explanation?</h5> | ||
| + | </html> | ||
<p align="justify"> | <p align="justify"> | ||
| - | Prcnr is a constitutive promoter, constantly producing RcnR protein which represses PrcnA. When there is nickel in the media RcnR alters its form, making it impossible to bind PrcnA, activating the production of the HoxN protein. This last protein is an efficient nickel transporter form '' | + | Prcnr is a constitutive promoter, constantly producing RcnR protein which represses PrcnA. When there is nickel in the media RcnR alters its form, making it impossible to bind PrcnA, activating the production of the HoxN protein. This last protein is an efficient nickel transporter form ''Cupriavidus metallidurans'' that places in the membrane allowing the income of the metal into the cell, it is one of the most efficent import nickel system reported in a bacterium <sup>4</sup>. |
| - | </ | + | <br><br> |
| - | < | + | |
Acording to the above, our system is recensusing for nickel everytime, and when this metal is detected, it is absorbed and consequently further increases its absorption. | Acording to the above, our system is recensusing for nickel everytime, and when this metal is detected, it is absorbed and consequently further increases its absorption. | ||
</p> | </p> | ||
| + | <html> | ||
<h5 id= "nickelChassis">Our chassis</h5> | <h5 id= "nickelChassis">Our chassis</h5> | ||
| + | </html> | ||
<p align="justify"> | <p align="justify"> | ||
| - | We chose ''Magnetospirillum magneticum'' AMB-1 as a final chassis this organism has the particularity to produce “magnetosomes” which are vesicles that contain 15 to 20 magnetite crystals that together act like a compass needle to orient magnetotactic bacteria in geomagnetic fields. This property, will allow us to eliminate the bacteria from water with a simple magnetic field. | + | We chose ''Magnetospirillum magneticum'' AMB-1 as a final chassis this organism has the particularity to produce “magnetosomes” which are vesicles that contain 15 to 20 magnetite crystals that together act like a compass needle to orient magnetotactic bacteria in geomagnetic fields<sup>5</sup>. This property, will allow us to eliminate the bacteria from water with a simple magnetic field. |
</p> | </p> | ||
| + | <html> | ||
<h5 id="nickelReporter">Reporter for PrcnA</h5> | <h5 id="nickelReporter">Reporter for PrcnA</h5> | ||
<p align="justify"> | <p align="justify"> | ||
| - | In order to check PrcnA obtained works in the presence of nickel, we will measure the | + | In order to check PrcnA obtained works in the presence of nickel, we will measure the mRFP activity, using our construct not only as a nickel removal system but as a quantitative nickel reporter as well. The color of mRFP is visible (this means, is not necessary a fluorometer to measure its production). |
</p> | </p> | ||
| - | <h5 id = "nickelProgress"> | + | </div> |
| + | </div> | ||
| + | </div> | ||
| + | <div class="accordion-group"> | ||
| + | <div class="accordion-heading"> | ||
| + | <a class="accordion-toggle" data-toggle="collapse" data-parent="#accordionhow" href="#collapseHow"> | ||
| + | How It works | ||
| + | </a> | ||
| + | </div> | ||
| + | <div id="collapseHow" class="accordion-body collapse"> | ||
| + | <div class="accordion-inner"> | ||
| + | </html> | ||
| + | [[File:howNicko.jpg]] | ||
| + | <html> | ||
| + | <ol> | ||
| + | <li>Several Nicko clones are producing in a bioreactor</li> | ||
| + | <li> Nicko is liberated to a "pool" where nickel contaminated water will be passing through</li> | ||
| + | <li> Nicko removes nickel from the water</li> | ||
| + | <li> Nicko is removed by a magnet</li> | ||
| + | <li> Water is now free of nicko and free of nickel</li> | ||
| + | </ol> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <div class="accordion-group"> | ||
| + | <div class="accordion-heading"> | ||
| + | <a class="accordion-toggle" data-toggle="collapse" data-parent="#accordion3" href="#collapseThree"> | ||
| + | Experimental work | ||
| + | </a> | ||
| + | </div> | ||
| + | <div id="collapseThree" class="accordion-body collapse"> | ||
| + | <div class="accordion-inner"> | ||
| + | <h5 id = "nickelProgress">Testing </html>''Magnetospirillum magneticum''<html> AMB-1 sensitivity to magnetic fields</h5> | ||
| + | </html> | ||
| + | |||
| + | <p align="justify"> | ||
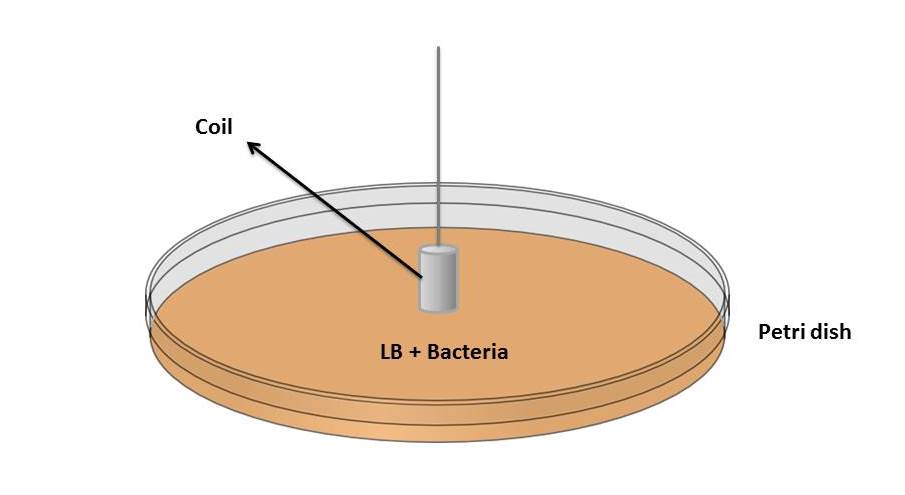
| + | As one of the major practical advantages of our system is the possibility of extracting the bacteria from the medium through magnetic fields, it was necessary to test the sensibility of ''Magnetospirillum magneticum'' AMB-1 to these fields. For this purpose, the following experimental procedure was developed: | ||
| + | </p> | ||
| + | |||
| + | 1. 1.5 ml of overnight culture was centrifuged at 8500 rpm for 5 min. Supernatant discarded. | ||
| + | |||
| + | 2. 10 ml of LB medium were added and then vortexed. | ||
| + | |||
| + | 3. A 100 uL aliquot was taken apart to proceed on performing the following assembly with the rest of the mixture. | ||
| + | |||
| + | [[File:Imgg.jpg |center | upright=3.0]] | ||
| + | |||
| + | 4. The coil was submitted to a 50 mA current source during 15 minutes (generating with this a field of about ten times the earth magnetic field). | ||
| + | |||
| + | 5. The coil was fastly transferred to 10 ml of water. | ||
| + | |||
| + | 6. The steps 2 and 5 samples were diluted and then spreaded at two different petri dishes (with LB + chloranphenicol) for later incubation at 37 ºC. | ||
| + | |||
| + | <p align="justify"> | ||
| + | After two days, the colonies of each simple were counted having 461 colonies in the sample obtained from the initial culture and 399 obtained from the bacteria attached to the coil, obtaining a recuperation percentage of 86,55%, thus showing the high sensitivity of ''Magnetospirillum magneticum'' AMB-1 to magnetic fields! | ||
| + | </p> | ||
| + | <p align="justify"> | ||
| + | We would like to thank professor Carlos Roberto Hernández and the magnetism laboratory for their support and advice during this procedure. | ||
| + | </p> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Here is a photo of the assembly: | ||
| + | </p> | ||
| + | |||
| + | [[File:Imgg2.jpg |center | upright=3.0]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <p align="justify"> | ||
| + | Finally you can find a recording of the experiment following this link: http://youtu.be/JnP_BjVqpQM | ||
| + | </p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
| - | </ | + | </div> |
| - | </ | + | </div> |
| + | </div> | ||
| + | <div class="accordion-group"> | ||
| + | <div class="accordion-heading"> | ||
| + | <a class="accordion-toggle" data-toggle="collapse" data-parent="#accordion4" href="#collapseFour"> | ||
| + | References | ||
| + | </a> | ||
| + | </div> | ||
| + | <div id="collapseFour" class="accordion-body collapse"> | ||
| + | <div class="accordion-inner"> | ||
| + | |||
| + | <h5 id= References">References</h5> | ||
| + | </html> | ||
| + | <p align="justify"> | ||
| + | [1] ''Nickel''. U.S. Geological Survey, Mineral Commodity Summaries. January 2013. | ||
| + | <br><br> | ||
| + | [2] Küpper, H. and Kroneck, P. ''Nickel in the Environment and its role in the metabolism of plants and cyanobacteria'' In Nickel and its surprising impact in Nature. Metal ions in life science, Vol. 2, Wiley 2007. | ||
| + | <br><br> | ||
| + | [3] Schaumlöffel, D. et al. '' J. Trace Elemn. Med. Bio.'' 2012, 26, 1-6. | ||
| + | <br><br> | ||
| + | [4] Eitinger, T. ''Microbial Nickel Transport'' In Microbial Transport Systems. Ed. G. Winkelmann. Wiley-VCH 2001. | ||
| + | <br><br> | ||
| + | [5] Blakemore, R. ''Science'' 1975, 190, 377–379. | ||
| + | </p> | ||
| + | <html> | ||
| + | |||
| + | <nowiki>* Updated as of September 24 </nowiki> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
| + | </div> | ||
| + | </div> | ||
</div> | </div> | ||
</html> | </html> | ||
Latest revision as of 03:41, 28 September 2013

Our Nickel Absorption System!
Nickel is a silvery-white, hard, malleable, and ductile metal. Now days the major use of nickel is in the preparation of alloys which are characterized by their strength, ductility, and resistance to corrosion and heat, such as stainless steel. It is also used for rechargeable batteries, catalysts and other chemicals among others. Most nickel on Earth is inaccessible; this is why the metal is mined in Russia, Australia, Cuba, Canada, Colombia, Venezuela and South Africa1.
As a result of this activity all kinds of water bodies are being contaminated with metals of this nature, Colombia, is one of the many affected. In small quantities nickel is essential, but when the uptake is too high it can be a dangerous to human health2,3 bringing many types of cancer, birth defects, abortions, asthma, allergic reactions such as skin rashes or “nickel-itch”, and many other conditions as its consequences.
Responding to these concerns, we have designed a bacterium which would be capable of absorbing the nickel in its interior using the internal regulating system of nickel belonging to the Cupriavidus metallidurans (called Ralstonia metallidurans before), to later be removed magnetically, using the property from Magnetospirillum magneticum AMB-1 which will be used as our final chassis.
Design: Project Parts!
Nickel Removal System
Our construct
Using the nickel homeostatic system of E. coli and Cupriavidus metallidurans, we will be able to create a system capable to detect and absorb nickel, to later be removed magnetically, using the magnetotactic property from Magnetospirillum magneticum AMB-1 which will be used as our final chassis.
Explanation?
Prcnr is a constitutive promoter, constantly producing RcnR protein which represses PrcnA. When there is nickel in the media RcnR alters its form, making it impossible to bind PrcnA, activating the production of the HoxN protein. This last protein is an efficient nickel transporter form Cupriavidus metallidurans that places in the membrane allowing the income of the metal into the cell, it is one of the most efficent import nickel system reported in a bacterium 4.
Acording to the above, our system is recensusing for nickel everytime, and when this metal is detected, it is absorbed and consequently further increases its absorption.
Our chassis
We chose Magnetospirillum magneticum AMB-1 as a final chassis this organism has the particularity to produce “magnetosomes” which are vesicles that contain 15 to 20 magnetite crystals that together act like a compass needle to orient magnetotactic bacteria in geomagnetic fields5. This property, will allow us to eliminate the bacteria from water with a simple magnetic field.
Reporter for PrcnA
In order to check PrcnA obtained works in the presence of nickel, we will measure the mRFP activity, using our construct not only as a nickel removal system but as a quantitative nickel reporter as well. The color of mRFP is visible (this means, is not necessary a fluorometer to measure its production).
Testing Magnetospirillum magneticum AMB-1 sensitivity to magnetic fields
As one of the major practical advantages of our system is the possibility of extracting the bacteria from the medium through magnetic fields, it was necessary to test the sensibility of Magnetospirillum magneticum AMB-1 to these fields. For this purpose, the following experimental procedure was developed:
1. 1.5 ml of overnight culture was centrifuged at 8500 rpm for 5 min. Supernatant discarded.
2. 10 ml of LB medium were added and then vortexed.
3. A 100 uL aliquot was taken apart to proceed on performing the following assembly with the rest of the mixture.
4. The coil was submitted to a 50 mA current source during 15 minutes (generating with this a field of about ten times the earth magnetic field).
5. The coil was fastly transferred to 10 ml of water.
6. The steps 2 and 5 samples were diluted and then spreaded at two different petri dishes (with LB + chloranphenicol) for later incubation at 37 ºC.
After two days, the colonies of each simple were counted having 461 colonies in the sample obtained from the initial culture and 399 obtained from the bacteria attached to the coil, obtaining a recuperation percentage of 86,55%, thus showing the high sensitivity of Magnetospirillum magneticum AMB-1 to magnetic fields!
We would like to thank professor Carlos Roberto Hernández and the magnetism laboratory for their support and advice during this procedure.
Here is a photo of the assembly:
Finally you can find a recording of the experiment following this link: http://youtu.be/JnP_BjVqpQM
References
[1] Nickel. U.S. Geological Survey, Mineral Commodity Summaries. January 2013.
[2] Küpper, H. and Kroneck, P. Nickel in the Environment and its role in the metabolism of plants and cyanobacteria In Nickel and its surprising impact in Nature. Metal ions in life science, Vol. 2, Wiley 2007.
[3] Schaumlöffel, D. et al. J. Trace Elemn. Med. Bio. 2012, 26, 1-6.
[4] Eitinger, T. Microbial Nickel Transport In Microbial Transport Systems. Ed. G. Winkelmann. Wiley-VCH 2001.
[5] Blakemore, R. Science 1975, 190, 377–379.
 "
"