Team:SJTU-BioX-Shanghai/Project/Regulator/Introdution
From 2013.igem.org
(→Key Parts of CRISPRi) |
|||
| (6 intermediate revisions not shown) | |||
| Line 29: | Line 29: | ||
| - | =From CRISPR to CRISPRi= | + | =<font color=white>From CRISPR to CRISPRi</font>= |
<br> | <br> | ||
<font size=4>CRISPR</font size=4> | <font size=4>CRISPR</font size=4> | ||
| Line 49: | Line 49: | ||
<br><br><br> | <br><br><br> | ||
| - | =Key Parts of CRISPRi= | + | =<font color=white>Key Parts of CRISPRi</font>= |
<br> | <br> | ||
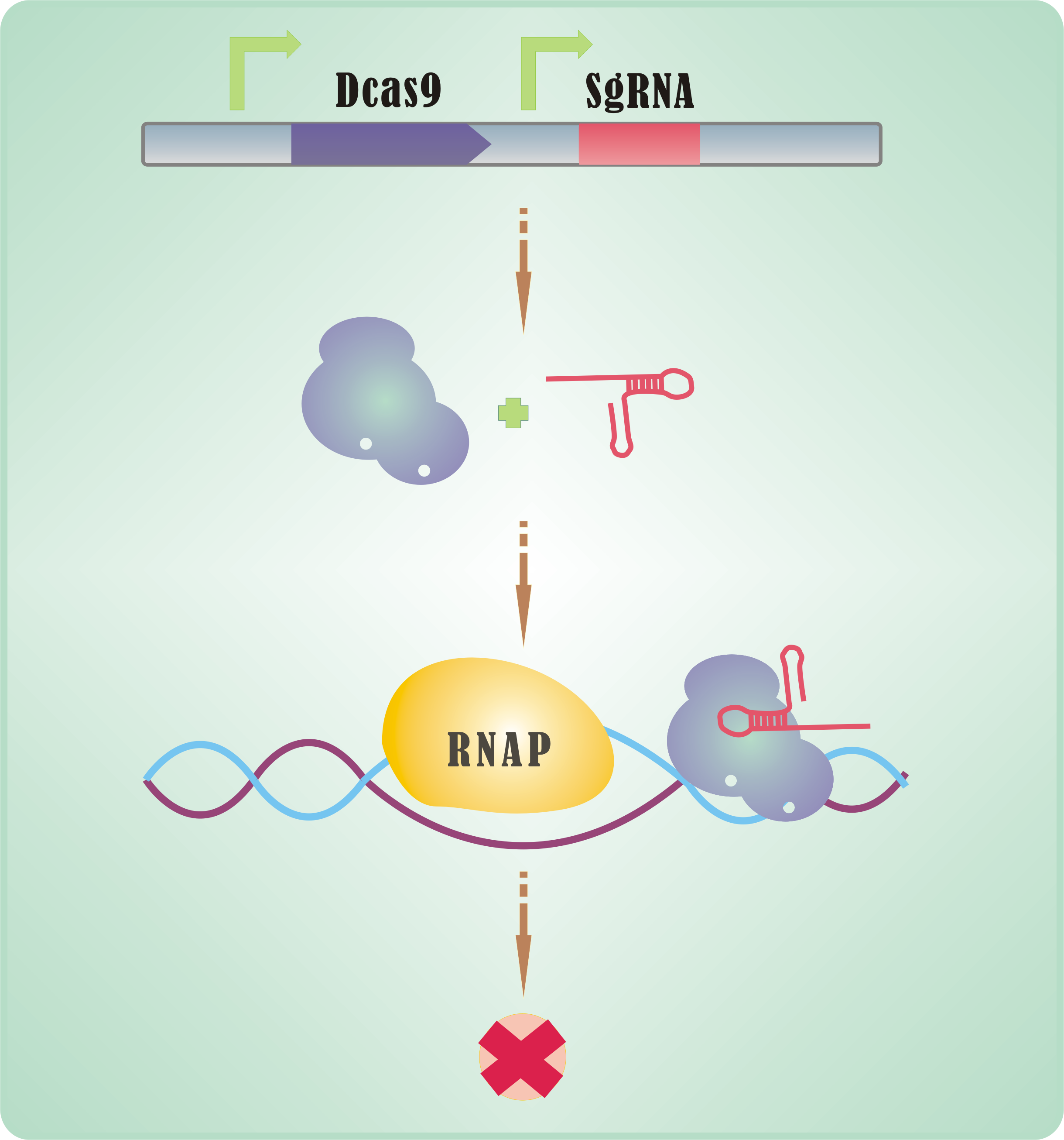
There are only 2 parts to be expressed -- dCas9 and sgRNA. Only <font size=4>2</font size=4>! | There are only 2 parts to be expressed -- dCas9 and sgRNA. Only <font size=4>2</font size=4>! | ||
| Line 61: | Line 61: | ||
[[File:SgRNA.png|thumb|250px|right|Structure of sgRNA]] | [[File:SgRNA.png|thumb|250px|right|Structure of sgRNA]] | ||
<br> | <br> | ||
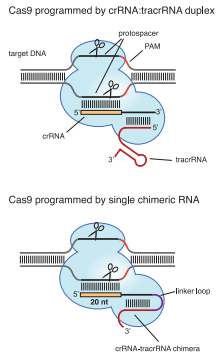
| - | If dCas9 acts as an executive, | + | If dCas9 acts as an executive, then sgRNA is the director of CRISPRi. |
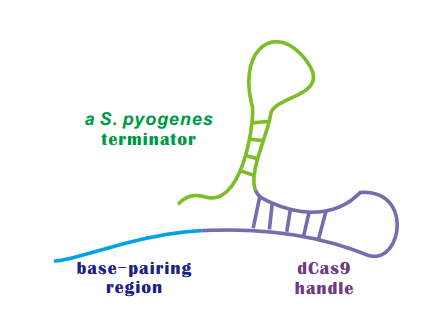
An sgRNA constitutes three parts: | An sgRNA constitutes three parts: | ||
* '''Base-Pairing Region''' | * '''Base-Pairing Region''' | ||
| Line 69: | Line 69: | ||
* A '''terminator''' derived from S. pyogenes, 42nt | * A '''terminator''' derived from S. pyogenes, 42nt | ||
<br> | <br> | ||
| - | + | <b><font size=2.5><font color=white>Design Criteria</font></font></b> (Qi et al., 2013) | |
* Binding specificity is determined by both sgRNA-DNA base pairing and a <b>protospacer adjacent motif (PAM), NGG</b>, upstream of target. | * Binding specificity is determined by both sgRNA-DNA base pairing and a <b>protospacer adjacent motif (PAM), NGG</b>, upstream of target. | ||
* Generally, the optimal length of the complementary region is <b>20nt</b>. | * Generally, the optimal length of the complementary region is <b>20nt</b>. | ||
| Line 81: | Line 81: | ||
<p style="color:grey;"> | <p style="color:grey;"> | ||
<br> | <br> | ||
| - | + | JINEK, M., CHYLINSKI, K., FONFARA, I., HAUER, M., DOUDNA, J. A. & CHARPENTIER, E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337, 816-821. | |
<br/><br/> | <br/><br/> | ||
| - | + | QI, LEI S., LARSON, MATTHEW H., GILBERT, LUKE A., DOUDNA, JENNIFER A., WEISSMAN, JONATHAN S., ARKIN, ADAM P. & LIM, WENDELL A. 2013. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell, 152, 1173-1183. | |
</p></html> | </p></html> | ||
Latest revision as of 21:23, 18 October 2013
|
| ||
|
 "
"