Team:Bielefeld-Germany/Project/Mediators
From 2013.igem.org
| (61 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
<html> | <html> | ||
<style> | <style> | ||
| - | h1{ | + | h1{margin-top:70px; } |
| - | + | ||
#globalwrapper ul {padding-left:40px; padding-right:40px;} | #globalwrapper ul {padding-left:40px; padding-right:40px;} | ||
| Line 17: | Line 17: | ||
h2,h3,h4{clear:both;} | h2,h3,h4{clear:both;} | ||
#globalwrapper h4{color:#ff6600; padding-left:20px;} | #globalwrapper h4{color:#ff6600; padding-left:20px;} | ||
| - | #globalwrapper div.thumb.tleft{margin-left: | + | #globalwrapper div.thumb.tleft{margin-left:20px; margin-right:20px; clear:both;} |
#globalwrapper ul {clear:both;} | #globalwrapper ul {clear:both;} | ||
| Line 49: | Line 49: | ||
<html> | <html> | ||
<h1>Mediators</h1> | <h1>Mediators</h1> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | <div | + | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:45px; clear:both;"> |
| - | + | <div class="bigbutton"> | |
| - | + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/MFC#Exogenous_Mediators">Exogenous<br> Mediators</a></p></div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <div class="bigbutton | + | |
| - | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/MFC">Exogenous Mediators</a></p></div> | + | |
<div class="bigbutton"> | <div class="bigbutton"> | ||
| - | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Riboflavine"> | + | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Riboflavine">Riboflavin</a></div> |
<div class="bigbutton"> | <div class="bigbutton"> | ||
<p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/GldA">Glycerol dehydrogenase</a></p></div> | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/GldA">Glycerol dehydrogenase</a></p></div> | ||
<div class="bigbutton"> | <div class="bigbutton"> | ||
<a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Phenazine">Phenazine</a></div> | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Project/Phenazine">Phenazine</a></div> | ||
| + | </div> | ||
</html> | </html> | ||
| - | |||
| + | ==Overview== | ||
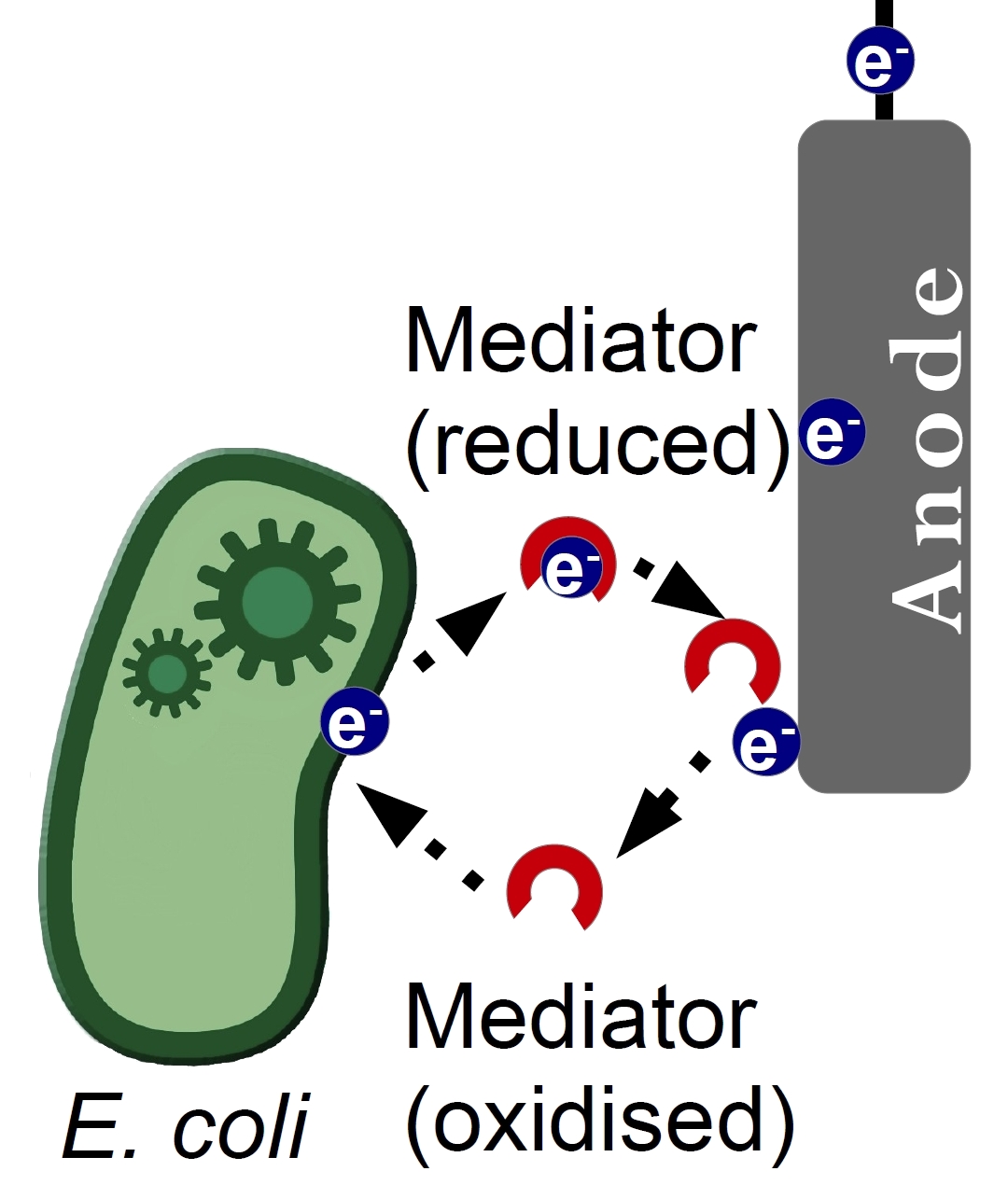
| - | + | [[Image:Bielefeld Germany Mediator principal.jpg|left|thumb|250px|'''Figure 1:''' Principle of electron transfer from bacteria to anode via mediators as electron shuttle.]] | |
| + | <p align="justify"> The efficiency of a Microbial Fuel Cell (MFC) depends on many factors. One of the known bottle necks is the electron transfer between a bacterium and the anode of the MFC. There are three mechanisms how this process occurs, through direct contact between bacterium and anode, through so-called nanowires and through a substance called a mediator. Mediators are usually small water-soluble molecules, that are able to undergo redox transformations. The mediator acts as an electron shuttle, enhancing the kinetics of the electron transfer. This approach has been proven to be generally quite successful and many substances were tested for their potential as electron shuttle.</p> | ||
| + | <br> | ||
| + | <p align="justify"> ''Escherichia coli'', the organism of our choice for use in the MFC, is not naturally able to transfer its electrons to the anode, so the usage of a mediator is crucial in our case. Due to their origin, mediators can be divided into two classes, the ones that are produced by a bacterium itself, so-called endogenous mediators, and the ones that are chemically synthesized and are added externally (exogenous mediators). The latter are very expensive, not completely degradable and to some extent even toxic for the environment. In some cases a special treatment is needed in order to catalyze their degradation process ([[Team:Bielefeld-Germany/Project/Mediators#References |Houas A ''et al''., 2001]]). In addition, exogenous mediators have to be added to the system repeatedly, because the active form of the molecule is short-lived in a MFC. All these aspects led to our decision to create an ''E. coli'' strain that will produce its own mediator. An endogenous mediator is environmentally neutral and does not have to be added externally, making it the optimal replacement by also saving cost.</p> | ||
| - | + | <br> | |
| + | <p align="justify"> We have chosen different targets for a genetic optimization, overproduction of glycerol dehydrogenase GldA for NADH generation and endogenous riboflavin synthesis (vitamin B2). We have also tried an overproduction of phenazine-1-carboxylic acid (PCA), but we had to realize that this substance is mainly known for its antibiotic properties. That raised some concerns, so we decided to concentrate our efforts on other sub-projects.</p> | ||
| + | <br> | ||
| + | To read more about each topic please refer to the corresponding pages on our Wiki. | ||
| + | *[[Team:Bielefeld-Germany/Project/MFC#Exogenous_Mediators |Exogenous mediators]] | ||
| + | *[[Team:Bielefeld-Germany/Project/Riboflavine |Riboflavin]] | ||
| + | *[[Team:Bielefeld-Germany/Project/GldA |Gylcerol dehydrogenase]] | ||
| + | *[[Team:Bielefeld-Germany/Project/Phenazine|Phenazine]] | ||
| - | + | <br><br> | |
| - | + | ||
===References=== | ===References=== | ||
| - | * | + | |
| + | *<p align="justify">Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. [http://www.sciencedirect.com/science/article/pii/S0734975007000547 ''Biotechnology advances'' 25(5): 464-482]. </p> | ||
| + | |||
| + | *<p align="justify">Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. [http://www.sciencedirect.com/science/article/pii/S0926337300002769 ''Applied Catalysis B: Environmental'' 31(2): 145-157]. </p> | ||
| + | |||
| + | *<p align="justify">Zhang T, Cui C, Chen S, Ai X, Yang H, Shen P, Peng Z (2006) A novel mediatorless microbial fuel cell based on direct biocatalysis of ''Escherichia coli''. [http://pubs.rsc.org/en/content/articlehtml/2006/cc/b600876c ''Chem Commun'' 11: 2257–2259''].</p> | ||
| + | |||
| Line 109: | Line 108: | ||
| - | <div id=" | + | |
| + | <div id="asdf"> | ||
| + | <html> | ||
| + | <div id="nav2" style="width:210px; padding-bottom:5px; padding-left:15px;"> | ||
| + | |||
| + | <div class="navbutton" id="home" style="float:left; padding-left:55px; margin-left:10px; padding-top:0px;"> | ||
| + | <a href="https://2013.igem.org/Team:Bielefeld-Germany" title="Jump to Frontpage"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f6/Bielefeld-Germany2013-ButtonHome.png" height="15px"> | ||
| + | </a> | ||
| + | </div> | ||
| + | <div class="navbutton" id="top" style="float:left; padding-left:10px; margin-left:10px; padding-top:0px;"> | ||
| + | <a href="#" title="Jump to top"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/a/ab/Bielefeld-Germany2013-Up_orange_new.png" height="15px"> | ||
| + | </a> | ||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <div id="rightcol" style="width:210px; height:100%; overflow-y:auto; box-shadow:0px 0px 2px 0px grey;" padding:0px 20px;> | ||
__TOC__ | __TOC__ | ||
| - | + | </div> | |
| + | |||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 15:49, 28 October 2013
Mediators
Overview
The efficiency of a Microbial Fuel Cell (MFC) depends on many factors. One of the known bottle necks is the electron transfer between a bacterium and the anode of the MFC. There are three mechanisms how this process occurs, through direct contact between bacterium and anode, through so-called nanowires and through a substance called a mediator. Mediators are usually small water-soluble molecules, that are able to undergo redox transformations. The mediator acts as an electron shuttle, enhancing the kinetics of the electron transfer. This approach has been proven to be generally quite successful and many substances were tested for their potential as electron shuttle.
Escherichia coli, the organism of our choice for use in the MFC, is not naturally able to transfer its electrons to the anode, so the usage of a mediator is crucial in our case. Due to their origin, mediators can be divided into two classes, the ones that are produced by a bacterium itself, so-called endogenous mediators, and the ones that are chemically synthesized and are added externally (exogenous mediators). The latter are very expensive, not completely degradable and to some extent even toxic for the environment. In some cases a special treatment is needed in order to catalyze their degradation process (Houas A et al., 2001). In addition, exogenous mediators have to be added to the system repeatedly, because the active form of the molecule is short-lived in a MFC. All these aspects led to our decision to create an E. coli strain that will produce its own mediator. An endogenous mediator is environmentally neutral and does not have to be added externally, making it the optimal replacement by also saving cost.
We have chosen different targets for a genetic optimization, overproduction of glycerol dehydrogenase GldA for NADH generation and endogenous riboflavin synthesis (vitamin B2). We have also tried an overproduction of phenazine-1-carboxylic acid (PCA), but we had to realize that this substance is mainly known for its antibiotic properties. That raised some concerns, so we decided to concentrate our efforts on other sub-projects.
To read more about each topic please refer to the corresponding pages on our Wiki.
References
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. [http://www.sciencedirect.com/science/article/pii/S0734975007000547 Biotechnology advances 25(5): 464-482].
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. [http://www.sciencedirect.com/science/article/pii/S0926337300002769 Applied Catalysis B: Environmental 31(2): 145-157].
Zhang T, Cui C, Chen S, Ai X, Yang H, Shen P, Peng Z (2006) A novel mediatorless microbial fuel cell based on direct biocatalysis of Escherichia coli. [http://pubs.rsc.org/en/content/articlehtml/2006/cc/b600876c Chem Commun 11: 2257–2259].
 "
"