Team:ETH Zurich/Experiments 7
From 2013.igem.org
| (96 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{:Team:ETH_Zurich/Templates/stylesheet}} | {{:Team:ETH_Zurich/Templates/stylesheet}} | ||
| - | <h1> | + | <h1>Reporter system</h1> |
| - | <p>Colisweeper depends | + | <p align="justify">Colisweeper depends on the [https://2013.igem.org/Team:ETH_Zurich/Infoproc processing of molecular signals] and generation of visible and distinguishable [https://2013.igem.org/Team:ETH_Zurich/Experiments_3 color outputs]. By using the wild type [https://2013.igem.org/Team:ETH_Zurich/Experiments_5 P<sub>LuxR</sub> promoter and mutant promoters] with varying AHL/LuxR affinities, gene expression can be induced for different concentrations of AHL. We coupled this system with reporter enzymes, called hydrolases, which catalyze hydrolysis of their colorless substrate into a colored product. Therefore, the player only triggers the colored response upon addition of a substrate. <br> |
| - | </p> | + | |
| + | |||
| + | The set of hydrolases we use include the ''Citrobacter'' <b>Alkaline phospohatase</b>, the ''Bacillus subtilis'' <b>β-Glucuronidase</b> and the ''Escherichia coli'' <b>Acetyl esterase</b>, <b>β-N-Acetylglucosaminidase</b> and <b>β-Galactosidase</b>. These enzymes have features that make them attractive as reporters. They are known to be relatively stable, exhibit activity under different conditions (e.g. pH and temperature), and catalyze the cleavage of various colorimetric and fluorescent substrates, which is ideal for visual screening. These hydrolases are all native to ''Escherichia coli'' and <b>orthogonal to each other</b> (see crosstalk experiment [https://2013.igem.org/Team:ETH_Zurich/Experiments_3#crosstalk here]), which means that each substrate can only be processed by the corresponding specific enzyme. In order to prevent background expression of these native enzymes, we use a [https://2013.igem.org/Team:ETH_Zurich/Mutant triple knockout strain] that has three hydrolase genes knocked out: ''uidA'' (β-glucuronidase), ''aes'' (acetyl esterase) and ''nagZ'' (β-N-acetylglucosaminidase). </p> | ||
| + | |||
| + | <p align="justify">Addition of a <b>multi-substrate mix</b> by the player leads to an enzyme-susbtrate reaction which specifically cleaves the chromogenic substrates, thereby producing a <b>visible color output</b>. Chromogenic substrates incorporate a chromophore whose absorbance properties change after the enzyme reaction, and the color signal produced then is directly related to the enzyme-catalyzed reaction ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 1]).</p> | ||
| + | |||
| + | [[File:Rainbow hydrolases.JPG|470px|right|thumb|<b>Figure 1: Colorimetric output.</b> This image shows liquid cultures of our mutant ''Escherichia coli'' strain expressing the enzymes PhoA, NagZ, LacZ, Aes or GusA to convert specific chromogenic substrates into colored outputs.]] | ||
| + | |||
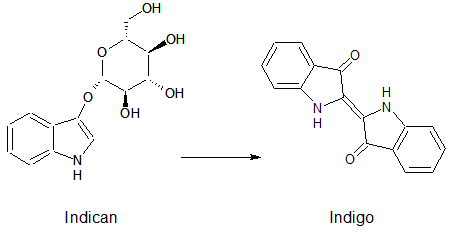
| + | <p align ="justify">Indican is a chromogenic glycoside hydrolase substrate which belongs to a family of natural glycosides found in plants. Cleavage of the glycosidic bond forms an unstable hydroxyindole intermediate, which dimerizes by oxidation to form indigo as a blue precipitate:</p> | ||
| + | [[File:IndicanIndigo.png|275px|center|]] | ||
| + | <p align="justify">Numerous enzyme substrates have been designed following this natural product example, giving rise to many colored phenols that are used to detect enzyme activities.<br><br> | ||
| + | Some of the hydrolases used in the Colisweeper reporter system can catalyze hydrolysis of various substrates, with different chromophores that give rise to a wide range of colors. This variety of substrates and colored outputs, additionally to the orthogonality of the enzyme-substrate reactions, enables usage of multiple hydrolases in reporter gene assays, which are becoming more important in real-time detection of cellular events associated with gene expression, regulation and signal transduction ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 2]). | ||
| + | |||
| + | <br><br> | ||
| + | |||
| + | For most parts encoding the hydrolases that were sent to the registry by our team, we have conducted fluorometric assays to study and compare their kinetics. The results can be seen <html><a href="https://2013.igem.org/Team:ETH_Zurich/Experiments_3" >here</a></html>, as well as in the experience section of the parts' registry sites, and the methods used are available <html><a href="https://2013.igem.org/Team:ETH_Zurich/Materials#enzyme_kinetics" >here</a></html>. | ||
| + | <br><br></p> | ||
<br clear="all"/> | <br clear="all"/> | ||
| - | < | + | <html><a id="hydrolase_aes" class="frog"></a></html> |
| - | < | + | <h1>Acetyl esterase (Aes)</h1> |
| - | + | ||
| - | < | + | [[File:Acetyl_esterase_3D.jpg|thumb|250px| 3D representation of the acetyl esterase from [http://www.rcsb.org/pdb/explore/explore.do?structureId=4KRX RCSB] ]] |
| - | + | <p align="justify">The ''Escherichia coli'' acetyl esterase is encoded by the ''aes'' gene and has been identified as a member of the hormone-sensitive lipase family ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 3]). Acetyl esterase interferes with the expression of the maltose system by counteracting maltose sensitivity ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 4]). This cytosolic enzyme is a 36 kDa monomer and catalyzes hydrolysis of short chain fatty esters with acyl chain lengths of up to eight carbons ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 3]). It is composed of 319 amino acid residues ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 5]) and its catalytic triad is composed of Ser165, Asp262 and His292 ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 6]). Various substrates can used for chromogenic enzyme assays with this enzyme, e.g. ''p''-nitrophenyl acetate ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 4]), ''p''-nitrophenyl butanoate ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 7]) and ''p''-nitrophenyl butyrate ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 8]). According to Kobayashi et al. ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 8]), kinetic constants for the ''Escherichia coli'' acetyl esterase are K<sub>m</sub> 170 ± 41 µM and k<sub>cat</sub> 29 ± 3.0 s<sup>-1</sup> when using ''p''-nitrophenyl butyrate as a substrate. This enzyme displays an optimal temperature at 65°C, depicting a specific activity of 250 U/mg using pNP-butanoate as substrate, but a low half-life at the same temperature (t<sub>1/2</sub> of inactivation = 5 minutes) ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 7]). <br> | |
| - | + | ||
| - | + | This enzyme's coding region has been added to the registry by our team as [http://parts.igem.org/Part:BBa_K1216002 BBa_K1216002]. We used this part in our final circuit and have made this our favorite part. Additionally, this part is available with an added TEV and poly-HIS tags for purification of the protein ([http://parts.igem.org/Part:BBa_K1216006 BBa_K1216006]). In Colisweeper, 5-Bromo-6-Chloro-3-indoxyl butyrate is used in the multi-mix substrate. After hydrolysis of this substrate, an indigo analog precipitates, which is visible as magenta color.</p> | |
| - | + | <br clear="all"/> | |
| - | + | ||
| - | In Colisweeper, 5-Bromo-6-Chloro-3-indoxyl butyrate is used in the multi-mix substrate. After hydrolysis of this substrate, an indigo analog precipitates, which | + | |
| - | < | + | |
| - | + | ||
| - | <h1> | + | <h1>Alkaline phosphatase (PhoA)</h1> |
| - | <p> | + | <html><a id="hydrolase_phoa" class="frog"></a></html> |
| - | + | ||
| + | [[File:Alkaline_Phosphatase_3D.jpg|thumb|left|250px| 3D representation of the alkaline phosphatase from [http://www.rcsb.org/pdb/explore/explore.do?structureId=1ANJ RCSB] ]] | ||
| + | <p align="justify"> | ||
| + | Alkaline phosphatase is a non-specific periplasmic phosphomonoesterase that catalyzes production of free inorganic phosphate and phosphoryl transfer reaction to various alcohols. In ''Escherichia coli'', this hydrolase is involved in the acquisition of phosphate from esters when free inorganic phosphate is scarce ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 9]), and is also capable of catalyzing the oxidation of phosphite to phosphate and molecular H<sub>2</sub> ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 10]).<br> | ||
| + | This enzyme is a homodimeric metalloenzyme with two Zn<sup>2+</sup> and on Mg<sup>2+</sup> forming the metal triplet at each active site, which is considered to be Asp101-Ser102-Ala103. It has been shown that with ''p''-nitrophenyl phosphate (pNPP) as a substrate, this enzyme has a specific activity of 1020 µmol pNPP h<sup>-1</sup> mg<sup>-1</sup> at pH 8; and its k<sub>cat</sub> and K<sub>m</sub> are 13.6 s<sup>-1</sup> and 7.4 µM, respectively ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 9]).<br> | ||
| + | In Colisweeper, the alkaline phosphatase is natively expressed in the ''Escherichia coli'' strain we used and processes pNPP of the substrate mix to produce yellow color. However, we made use of the ''Citrobacter'' ''phoA'' gene ([http://parts.igem.org/Part:BBa_K1216001 BBa_K1216001]) for kinetics assays of this enzyme. Also, we have added an improved version of this part to the registry ([http://parts.igem.org/Part:BBa_K1216005 BBa_K1216005]), with TEV and HIS-tags added to enable protein purification.</p> | ||
| + | <br clear="all"/> | ||
<h1>β-Galactosidase (LacZ)</h1> | <h1>β-Galactosidase (LacZ)</h1> | ||
| - | < | + | <html><a id="hydrolase_lacz" class="frog"></a></html> |
| - | <br> | + | [[File:galactosidase_3d_model.jpg|thumb|250px| 3D representation of the β-Galactosidase from [http://www.rcsb.org/pdb/explore/explore.do?structureId=3VD5 RCSB] ]] |
| + | <p align="justify">The ''Escherichia coli'' β-galactosidase is encoded by the gene ''lacZ'', a part of the ''lac'' operon which is inducible by lactose in the absence of glucose. β-Galactosidase catalyzes hydrolysis of glycosidic bonds between galactose and its moiety ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 11]). It is commonly used as a reporter to indicate gene expression by using a chromogenic substrate for detection e.g. X-Gal, which forms a blue product after cleavage.<br> | ||
| + | This enzyme is natively expressed in the ''Escherichia coli'' strain used for Colisweeper. Its function in this game is to catalyze hydrolysis of the flagging substrate, β-D-Green-Gal, which results in the formation of green color output.</p> | ||
| + | <br clear="all"/> | ||
| + | |||
| + | <h1>β-Glucuronidase (GusA)</h1> | ||
| + | <html><a id="hydrolase_gusa" class="frog"></a></html> | ||
| + | |||
| + | [[File:Glucuronidase_3d_model.jpg|thumb|left|250px| 3D representation of the β-Glucuronidase from [http://www.rcsb.org/pdb/explore/explore.do?structureId=3K46 RCSB] ]] | ||
| + | <p align="justify"> | ||
| + | The enzyme β-glucuronidase catalyzes the hydrolysis of D-glucuronic acids which are conjugated through a β-O-glycosidic linkage to an aglycone. The action of bacterial β-glucuronidase in the gastrointestinal tract is an important component in the enterohepatic circulation of many hydrophobic xenobiotics and endogenous waste compounds, which are conjugated to D-glucuronic acid during the main detoxification pathway of vertebrates, and are then excreted in the bile or urine ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 12]). The reduction of these beta-d-glucuronides by β-glucuronidase activity frees aglycone residues with protective effects, such as lignans, flavonoids, ceramide and glycyrrhetinic acid ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 13]), enabling their absorption and enterohepatic circulation.<br> | ||
| + | β-Glucuronidase is commonly used as a reporter system for various experimental measurements, and has been favoured as a reporter gene in plants. In contrast to β-galactosidase, which is a widely used reporter protein, β-glucuronidase has a key advantage in its robustness and its absence in many organisms other than vertebrates and their attendant microflora, rendering the possibility of accurate measurements of β-glucuronidase activity ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 14]). <br> | ||
| + | The part we implemented in Colisweeper is ''gusA'' ([http://parts.igem.org/Part:BBa_K1216000 BBa_K1216000]), a homolog of the ''Escherichia coli'' ''uidA'', and encodes the ''Bacillus subtilis'' β-glucuronidase, a 68 kDa tetrameric and intracellular enzyme. This part is also available with added TEV and poly-HIS tags ([http://parts.igem.org/Part:BBa_K1216004 BBa_K1216004]). According to Tryland and Fiksdal ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 15]), the activity of the ''Bacillus spp.'' β-glucuronidase is similar to that of the ''Escherichia coli'' homolog, being only approximately 10-fold lower. In our game, β-glucuronidase catalyzes hydrolysis of 6-Chloro-3-indolyl-β-glucuronic acid, which gives rise to an orange-pink color.</p><br> | ||
| + | <br clear="all"/> | ||
| + | |||
| + | |||
| + | <h1>β-N-Acetylglucosaminidase (NagZ)</h1> | ||
| + | <html><a id="hydrolase_nagz" class="frog"></a></html> | ||
| + | |||
| + | [[File:β-N-Acetylglucosaminidase_3D.jpg|thumb|250px| 3D representation of the β-N-Acetylglucosaminidase from [http://www.rcsb.org/pdb/explore/explore.do?structureId=4GVG RCSB] ]] | ||
| + | <p align="justify"> The β-N-Acetylglucosaminidase enzyme is a cytoplasmic hydrolase which is involved in the ''Escherichia coli'' cell wall recycling pathway. Its natural substrates are muropeptides and anhydro-muropeptides, which are released into the cytosol from cell wall murein ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 16]). The enzyme is active against the β-1,4-glycosidic bonds and releases β-N-acetylglucosamine ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 17]). Inhibitors of NagZ are N-acetylglucosamine and N-acetylmuramic acid at high concentrations, or N-acetylglucosaminolactone ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 17]). Using p-nitrophenyl-β-N-acetylglucosaminide as a substrate, a kinetic assay by Vötsch and Templin ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7#hydrolase_reference 18]) showed a K<sub>m</sub> 310 µM and V<sub>max</sub> 13.3 µmol/min mg protein at 25°C.<br> | ||
| + | We have added the coding region for β-N-Acetylglucosaminidase to the registry as [http://parts.igem.org/Part:BBa_K1216003 BBa_K1216003] and used this part for Colisweeper's final circuit in the mine cells, which produce blue color upon hydrolysis of the substrate 5-Bromo-4-Chloro-3-indoxyl-β-N-acetylglucosaminide. | ||
| + | </p> | ||
| + | <br clear="all"/> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | <html><a id="hydrolase_reference" class="frog"></a></html> | ||
<h1>References</h1> | <h1>References</h1> | ||
| - | (1) Kanaya S, Koyanagi T, Kanaya E, Biochem. J., 332, 75-80 (1998).<br> | + | (1) Reymond JL, Fluxà VS, Maillard N, ''Chem. Commun.'', '''1''', 34 – 46 (2009).<br> |
| - | ( | + | (2) Jiang T, Xing B, Rao J, ''Biotechnology and Genetic Engineering Reviews'', '''25''', 41-76 (2008).<br> |
| - | ( | + | (3) Kanaya S, Koyanagi T, Kanaya E, ''Biochem. J.'', '''332''', 75-80 (1998).<br> |
| - | ( | + | (4) Peist R, Koch A, Bolek P, Sewitz S, Kolbus T, Boos J, ''J. Bacteriol.'', '''179''', 7679-7686 (1997).<br> |
| - | ( | + | (5) Blattner FR, Plunkett III G, Bloch CA, Perna NT, Burna V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y, ''Science'', '''277''', 1453-1461 (1997).<br> |
| - | ( | + | (6) Haruki M, Oohashi Y, Mizuguchi S, Matsuo Y, Morikawa M, Kanaya S, ''FEBS Lett.'', '''454''', 262-266 (1999).<br> |
| + | (7) Farias T, Mandrich L, Rossi M, Manco G, ''Protein Pept. Lett.'', '''14''', 165-169 (2007).<br> | ||
| + | (8) Kobayashi R, Hirano N, Kanaya S, Haruki M, ''Biosci. Biotechnol. Biochem.'', '''76''' (11), 2082-2088 (2012).<br> | ||
| + | (9) Kim EE, Wyckoff HW, ''J. Mol. Biol.'', '''218''', 449-464 (1991).<br> | ||
| + | (10) Yang K, Metcalf WW, ''PNAS'', '''101''', 7919-7924 (2004).<br> | ||
| + | (11) Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW, ''Biochemistry'', '''40''', 14781-14794 (2001).<br> | ||
| + | (12) Russell WM, Klaenhammer TR, ''Appl. Environ. Microbiol.'', '''67''', 1253-1261 (2000).<br> | ||
| + | (13) Beaud D, Tailliez P, Anba-Mondoloni J, ''Microbiology'', '''171''', 2323-2330 (2005).<br> | ||
| + | (14) Jefferson RA, Burgess SM, Hirsh D, ''Procl. Natl. Acad. Sci. USA'', '''83''', 8447-8451 (1986).<br> | ||
| + | (15) Tryland I, Fiksdal L, ''Appl. Environ. Microbiol.'', '''64''', 1018-1023 (1997).<br> | ||
| + | (16) Cheng Q, Li H, Merdek K, Park JT, ''J. Bacteriol.'', '''182''', 4836-4840 (2000).<br> | ||
| + | (17) Yem DW, Wu HC, ''J. Bacteriol.'', '''125''', 324-331 (1976).<br> | ||
| + | (18) Vötsch W, Templin MF, ''J. Biol. Chem.'', '''275''', 39032-39038 (2000).<br> | ||
| + | |||
<br clear="all"/> | <br clear="all"/> | ||
{{:Team:ETH_Zurich/templates/footer}} | {{:Team:ETH_Zurich/templates/footer}} | ||
Latest revision as of 23:37, 28 October 2013
Contents |
Reporter system
Colisweeper depends on the processing of molecular signals and generation of visible and distinguishable color outputs. By using the wild type PLuxR promoter and mutant promoters with varying AHL/LuxR affinities, gene expression can be induced for different concentrations of AHL. We coupled this system with reporter enzymes, called hydrolases, which catalyze hydrolysis of their colorless substrate into a colored product. Therefore, the player only triggers the colored response upon addition of a substrate.
The set of hydrolases we use include the Citrobacter Alkaline phospohatase, the Bacillus subtilis β-Glucuronidase and the Escherichia coli Acetyl esterase, β-N-Acetylglucosaminidase and β-Galactosidase. These enzymes have features that make them attractive as reporters. They are known to be relatively stable, exhibit activity under different conditions (e.g. pH and temperature), and catalyze the cleavage of various colorimetric and fluorescent substrates, which is ideal for visual screening. These hydrolases are all native to Escherichia coli and orthogonal to each other (see crosstalk experiment here), which means that each substrate can only be processed by the corresponding specific enzyme. In order to prevent background expression of these native enzymes, we use a triple knockout strain that has three hydrolase genes knocked out: uidA (β-glucuronidase), aes (acetyl esterase) and nagZ (β-N-acetylglucosaminidase).
Addition of a multi-substrate mix by the player leads to an enzyme-susbtrate reaction which specifically cleaves the chromogenic substrates, thereby producing a visible color output. Chromogenic substrates incorporate a chromophore whose absorbance properties change after the enzyme reaction, and the color signal produced then is directly related to the enzyme-catalyzed reaction (1).
Indican is a chromogenic glycoside hydrolase substrate which belongs to a family of natural glycosides found in plants. Cleavage of the glycosidic bond forms an unstable hydroxyindole intermediate, which dimerizes by oxidation to form indigo as a blue precipitate:
Numerous enzyme substrates have been designed following this natural product example, giving rise to many colored phenols that are used to detect enzyme activities.
Some of the hydrolases used in the Colisweeper reporter system can catalyze hydrolysis of various substrates, with different chromophores that give rise to a wide range of colors. This variety of substrates and colored outputs, additionally to the orthogonality of the enzyme-substrate reactions, enables usage of multiple hydrolases in reporter gene assays, which are becoming more important in real-time detection of cellular events associated with gene expression, regulation and signal transduction (2).
For most parts encoding the hydrolases that were sent to the registry by our team, we have conducted fluorometric assays to study and compare their kinetics. The results can be seen here, as well as in the experience section of the parts' registry sites, and the methods used are available here.
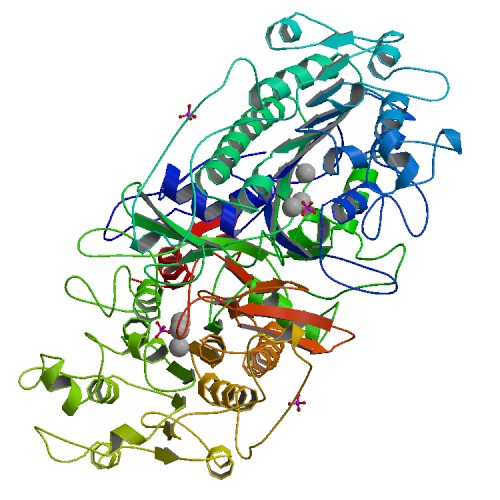
Acetyl esterase (Aes)
The Escherichia coli acetyl esterase is encoded by the aes gene and has been identified as a member of the hormone-sensitive lipase family (3). Acetyl esterase interferes with the expression of the maltose system by counteracting maltose sensitivity (4). This cytosolic enzyme is a 36 kDa monomer and catalyzes hydrolysis of short chain fatty esters with acyl chain lengths of up to eight carbons (3). It is composed of 319 amino acid residues (5) and its catalytic triad is composed of Ser165, Asp262 and His292 (6). Various substrates can used for chromogenic enzyme assays with this enzyme, e.g. p-nitrophenyl acetate (4), p-nitrophenyl butanoate (7) and p-nitrophenyl butyrate (8). According to Kobayashi et al. (8), kinetic constants for the Escherichia coli acetyl esterase are Km 170 ± 41 µM and kcat 29 ± 3.0 s-1 when using p-nitrophenyl butyrate as a substrate. This enzyme displays an optimal temperature at 65°C, depicting a specific activity of 250 U/mg using pNP-butanoate as substrate, but a low half-life at the same temperature (t1/2 of inactivation = 5 minutes) (7).
This enzyme's coding region has been added to the registry by our team as [http://parts.igem.org/Part:BBa_K1216002 BBa_K1216002]. We used this part in our final circuit and have made this our favorite part. Additionally, this part is available with an added TEV and poly-HIS tags for purification of the protein ([http://parts.igem.org/Part:BBa_K1216006 BBa_K1216006]). In Colisweeper, 5-Bromo-6-Chloro-3-indoxyl butyrate is used in the multi-mix substrate. After hydrolysis of this substrate, an indigo analog precipitates, which is visible as magenta color.
Alkaline phosphatase (PhoA)
Alkaline phosphatase is a non-specific periplasmic phosphomonoesterase that catalyzes production of free inorganic phosphate and phosphoryl transfer reaction to various alcohols. In Escherichia coli, this hydrolase is involved in the acquisition of phosphate from esters when free inorganic phosphate is scarce (9), and is also capable of catalyzing the oxidation of phosphite to phosphate and molecular H2 (10).
This enzyme is a homodimeric metalloenzyme with two Zn2+ and on Mg2+ forming the metal triplet at each active site, which is considered to be Asp101-Ser102-Ala103. It has been shown that with p-nitrophenyl phosphate (pNPP) as a substrate, this enzyme has a specific activity of 1020 µmol pNPP h-1 mg-1 at pH 8; and its kcat and Km are 13.6 s-1 and 7.4 µM, respectively (9).
In Colisweeper, the alkaline phosphatase is natively expressed in the Escherichia coli strain we used and processes pNPP of the substrate mix to produce yellow color. However, we made use of the Citrobacter phoA gene ([http://parts.igem.org/Part:BBa_K1216001 BBa_K1216001]) for kinetics assays of this enzyme. Also, we have added an improved version of this part to the registry ([http://parts.igem.org/Part:BBa_K1216005 BBa_K1216005]), with TEV and HIS-tags added to enable protein purification.
β-Galactosidase (LacZ)
The Escherichia coli β-galactosidase is encoded by the gene lacZ, a part of the lac operon which is inducible by lactose in the absence of glucose. β-Galactosidase catalyzes hydrolysis of glycosidic bonds between galactose and its moiety (11). It is commonly used as a reporter to indicate gene expression by using a chromogenic substrate for detection e.g. X-Gal, which forms a blue product after cleavage.
This enzyme is natively expressed in the Escherichia coli strain used for Colisweeper. Its function in this game is to catalyze hydrolysis of the flagging substrate, β-D-Green-Gal, which results in the formation of green color output.
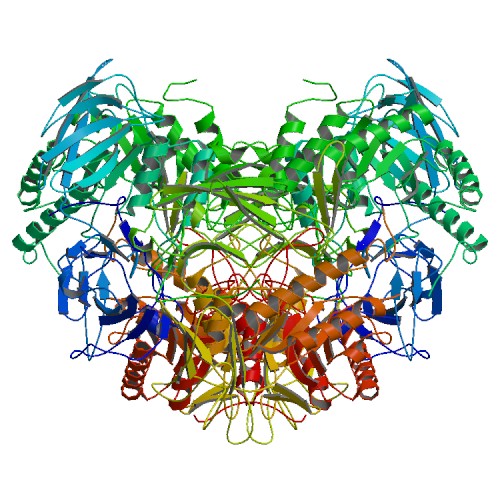
β-Glucuronidase (GusA)
The enzyme β-glucuronidase catalyzes the hydrolysis of D-glucuronic acids which are conjugated through a β-O-glycosidic linkage to an aglycone. The action of bacterial β-glucuronidase in the gastrointestinal tract is an important component in the enterohepatic circulation of many hydrophobic xenobiotics and endogenous waste compounds, which are conjugated to D-glucuronic acid during the main detoxification pathway of vertebrates, and are then excreted in the bile or urine (12). The reduction of these beta-d-glucuronides by β-glucuronidase activity frees aglycone residues with protective effects, such as lignans, flavonoids, ceramide and glycyrrhetinic acid (13), enabling their absorption and enterohepatic circulation.
β-Glucuronidase is commonly used as a reporter system for various experimental measurements, and has been favoured as a reporter gene in plants. In contrast to β-galactosidase, which is a widely used reporter protein, β-glucuronidase has a key advantage in its robustness and its absence in many organisms other than vertebrates and their attendant microflora, rendering the possibility of accurate measurements of β-glucuronidase activity (14).
The part we implemented in Colisweeper is gusA ([http://parts.igem.org/Part:BBa_K1216000 BBa_K1216000]), a homolog of the Escherichia coli uidA, and encodes the Bacillus subtilis β-glucuronidase, a 68 kDa tetrameric and intracellular enzyme. This part is also available with added TEV and poly-HIS tags ([http://parts.igem.org/Part:BBa_K1216004 BBa_K1216004]). According to Tryland and Fiksdal (15), the activity of the Bacillus spp. β-glucuronidase is similar to that of the Escherichia coli homolog, being only approximately 10-fold lower. In our game, β-glucuronidase catalyzes hydrolysis of 6-Chloro-3-indolyl-β-glucuronic acid, which gives rise to an orange-pink color.
β-N-Acetylglucosaminidase (NagZ)
The β-N-Acetylglucosaminidase enzyme is a cytoplasmic hydrolase which is involved in the Escherichia coli cell wall recycling pathway. Its natural substrates are muropeptides and anhydro-muropeptides, which are released into the cytosol from cell wall murein (16). The enzyme is active against the β-1,4-glycosidic bonds and releases β-N-acetylglucosamine (17). Inhibitors of NagZ are N-acetylglucosamine and N-acetylmuramic acid at high concentrations, or N-acetylglucosaminolactone (17). Using p-nitrophenyl-β-N-acetylglucosaminide as a substrate, a kinetic assay by Vötsch and Templin (18) showed a Km 310 µM and Vmax 13.3 µmol/min mg protein at 25°C.
We have added the coding region for β-N-Acetylglucosaminidase to the registry as [http://parts.igem.org/Part:BBa_K1216003 BBa_K1216003] and used this part for Colisweeper's final circuit in the mine cells, which produce blue color upon hydrolysis of the substrate 5-Bromo-4-Chloro-3-indoxyl-β-N-acetylglucosaminide.
References
(1) Reymond JL, Fluxà VS, Maillard N, Chem. Commun., 1, 34 – 46 (2009).
(2) Jiang T, Xing B, Rao J, Biotechnology and Genetic Engineering Reviews, 25, 41-76 (2008).
(3) Kanaya S, Koyanagi T, Kanaya E, Biochem. J., 332, 75-80 (1998).
(4) Peist R, Koch A, Bolek P, Sewitz S, Kolbus T, Boos J, J. Bacteriol., 179, 7679-7686 (1997).
(5) Blattner FR, Plunkett III G, Bloch CA, Perna NT, Burna V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y, Science, 277, 1453-1461 (1997).
(6) Haruki M, Oohashi Y, Mizuguchi S, Matsuo Y, Morikawa M, Kanaya S, FEBS Lett., 454, 262-266 (1999).
(7) Farias T, Mandrich L, Rossi M, Manco G, Protein Pept. Lett., 14, 165-169 (2007).
(8) Kobayashi R, Hirano N, Kanaya S, Haruki M, Biosci. Biotechnol. Biochem., 76 (11), 2082-2088 (2012).
(9) Kim EE, Wyckoff HW, J. Mol. Biol., 218, 449-464 (1991).
(10) Yang K, Metcalf WW, PNAS, 101, 7919-7924 (2004).
(11) Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW, Biochemistry, 40, 14781-14794 (2001).
(12) Russell WM, Klaenhammer TR, Appl. Environ. Microbiol., 67, 1253-1261 (2000).
(13) Beaud D, Tailliez P, Anba-Mondoloni J, Microbiology, 171, 2323-2330 (2005).
(14) Jefferson RA, Burgess SM, Hirsh D, Procl. Natl. Acad. Sci. USA, 83, 8447-8451 (1986).
(15) Tryland I, Fiksdal L, Appl. Environ. Microbiol., 64, 1018-1023 (1997).
(16) Cheng Q, Li H, Merdek K, Park JT, J. Bacteriol., 182, 4836-4840 (2000).
(17) Yem DW, Wu HC, J. Bacteriol., 125, 324-331 (1976).
(18) Vötsch W, Templin MF, J. Biol. Chem., 275, 39032-39038 (2000).
 "
"