Team:ETH Zurich/Modeling/InitialStates
From 2013.igem.org
| (13 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
<h1> Initial state for the Mine Cells </h1> | <h1> Initial state for the Mine Cells </h1> | ||
| - | <p align= "justify">The mine colonies produce three molecules, NagZ, LuxI and AHL, that are important in our bio-game. The proteins NagZ and LuxI are constitutively produced, whereas the synthesis of AHL is dependent | + | <p align= "justify">The mine colonies produce three molecules, NagZ, LuxI and AHL, that are important in our bio-game. The proteins NagZ and LuxI are constitutively produced, whereas the synthesis of AHL is dependent on LuxI. Moreover, degradation of these proteins is modeled as linear degradation. We have also taken into account the contribution of the dynamic evolution of AHL due to diffusion toward/from the medium, distinguishing between intracellular and extracellular concentrations ([https://2013.igem.org/Team:ETH_Zurich/References#Garcia2004 Garcia-Ojalvo ''et. al.'', 2004.])<br><br> |
The ODEs for the states involved in the mine cells are given below: <br><br></p> | The ODEs for the states involved in the mine cells are given below: <br><br></p> | ||
| Line 50: | Line 50: | ||
[AHL,e]_{ss}= \frac{ \eta_{ext} \cdot [AHL,i]_{ss}}{d_{AHL,e} + \eta_{ext} } \approx 55.8291 \mu M | [AHL,e]_{ss}= \frac{ \eta_{ext} \cdot [AHL,i]_{ss}}{d_{AHL,e} + \eta_{ext} } \approx 55.8291 \mu M | ||
\end{align} | \end{align} | ||
| - | <h1> Initial state for the Receiver Cells </h1> | + | <h1> Initial state for the Receiver Cells</h1> |
| - | <p align= "justify">It should be noted that the receiver cells grow in the absence of AHL in the medium and this affects the concentrations of intracellular species whose production depends on AHL.</p> | + | <p align= "justify"> Receiver cells are responsible of processing the AHL signal and generating a visible distinguishable colour outputs. To do so, we introduce a enzymatic reporter system using the hydrolases GusA and AES. Proteins are expressed under the control of P<sub>LuxR</sub> promoters which are activated by LuxR/AHL complex (denoted as r). We used the wild type promoter to drive expression of GusA and a promoter mutant, less sensitive towards LuxR/AHL complex, for AES. |
| - | {|style="border: none;" align="center" | + | |
| - | |valign="top"|[[File:InitialConReceiver.png|500px|center|thumb|<b>Figure 2:</b> Steady state concentrations for Receiver Cells.]] | + | The ODEs for the states involved in the receiver cells are given below: <br><br></p> |
| - | |valign="top"|[[File:InitialConReceiver2.png|500px|center|thumb|<b>Figure 3:</b> Steady state concentrations for Receiver Cells]] | + | |
| + | \begin{align} | ||
| + | \frac{d[LuxR]}{dt}=\alpha_{[LuxR]} - d_{LuxR} [LuxR] \\ | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | \frac{d[R]}{dt}=\rho_{LuxR} \cdot [LuxR ]^2 \cdot [AHL]^2 - d_{R} [R]\\ | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | \frac{d[AHL,i]}{dt}= \eta \cdot \left([AHL,e]-[AHL,i]\right) -d_{AHL,i} \cdot [AHL,i]\\ | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | \frac{d[AHL,e]}{dt}= -d_{AHL,e} \cdot [AHL,e] + \eta_{ext} \sum_{j=1}^{n} \left([AHL,j]-[AHL,e]\right)\\ | ||
| + | \end{align} | ||
| + | |||
| + | |||
| + | \begin{align} | ||
| + | \frac{d[GusA]}{dt}=\alpha_{GusA} \cdot k_{leaky} \cdot [LuxR]+ \frac{\alpha_{GusA}(\frac{[R]}{K_{R_1}})^{n}}{1+(\frac{[R]}{K_{R_1}})^{n}} - d_{GusA}\cdot [GusA]\\ | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | \frac{d[AES]}{dt}=\alpha_{AES} \cdot k_{leaky} \cdot [LuxR] + \frac{\alpha_{AES}(\frac{[R]}{K_{R_2}})^{n}}{1+(\frac{[R]}{K_{R_2}})^{n}} - d_{AES}\cdot [AES]\\ | ||
| + | \end{align} | ||
| + | |||
| + | <p align="justify"> It should be noted that the receiver cells grow in the absence of AHL in the medium and this affects the concentrations of intracellular species whose production depends on AHL.</p> | ||
| + | |||
| + | {|style="border: none;background: transparent;" align="center" | ||
| + | |valign="top" style="border:none;"|[[File:InitialConReceiver.png|500px|center|thumb|<b>Figure 2:</b> Steady state concentrations for Receiver Cells.]] | ||
| + | |valign="top" style="border:none;"|[[File:InitialConReceiver2.png|500px|center|thumb|<b>Figure 3:</b> Steady state concentrations for Receiver Cells]] | ||
|} | |} | ||
| + | |||
| + | <br> | ||
| + | <p align= "justify"> The analytical solution for the steady state is given below: </p> | ||
| + | |||
| + | \begin{align} | ||
| + | [LuxR]_{ss}= \frac{\alpha_{LuxR}}{d_{LuxR}} \approx 0.5 \mu M | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | [GusA]_{ss}= \frac{\alpha_{GusA} \cdot k_{leaky} \cdot [LuxR]_{ss}}{d_{GusA}} \approx 3.2461 \mu M | ||
| + | \end{align} | ||
| + | |||
| + | \begin{align} | ||
| + | [AES]_{ss}= \frac{\alpha_{AES} \cdot k_{leaky} \cdot [LuxR]_{ss}}{d_{AES}} \approx 3.2461 \mu M | ||
| + | \end{align} | ||
| + | |||
| + | <h1> Output System </h1> | ||
| + | |||
| + | <p align= "justify">GusA expression should be triggered by one mine in the vicinity. In figure 4 is shown the GusA steady state concentration as function of AHL as input. We simulated the system of equations for 5000 minutes. </p> <br> | ||
| + | [[File:GusA_AHLsweep.png|600px|center|thumb|<b>Figure 4:</b> GusA concentration vs. increasing AHL input, both in μ M.]] | ||
<br clear="all"/> | <br clear="all"/> | ||
{{:Team:ETH_Zurich/templates/footer}} | {{:Team:ETH_Zurich/templates/footer}} | ||
Latest revision as of 23:11, 28 October 2013
Contents |
Initial States
It is important to obtain the steady state concentration of molecules in our biological circuit, since these values can be used as biologically meaningful initial conditions for subsequent simulations. To achieve this goal, we implemented a Single Cell Model. The cells plated on the game plate are from an overnight liquid culture. Thus, we assume that the levels of the proteins of our engineered system in the cells have reached steady state.
Initial state for the Mine Cells
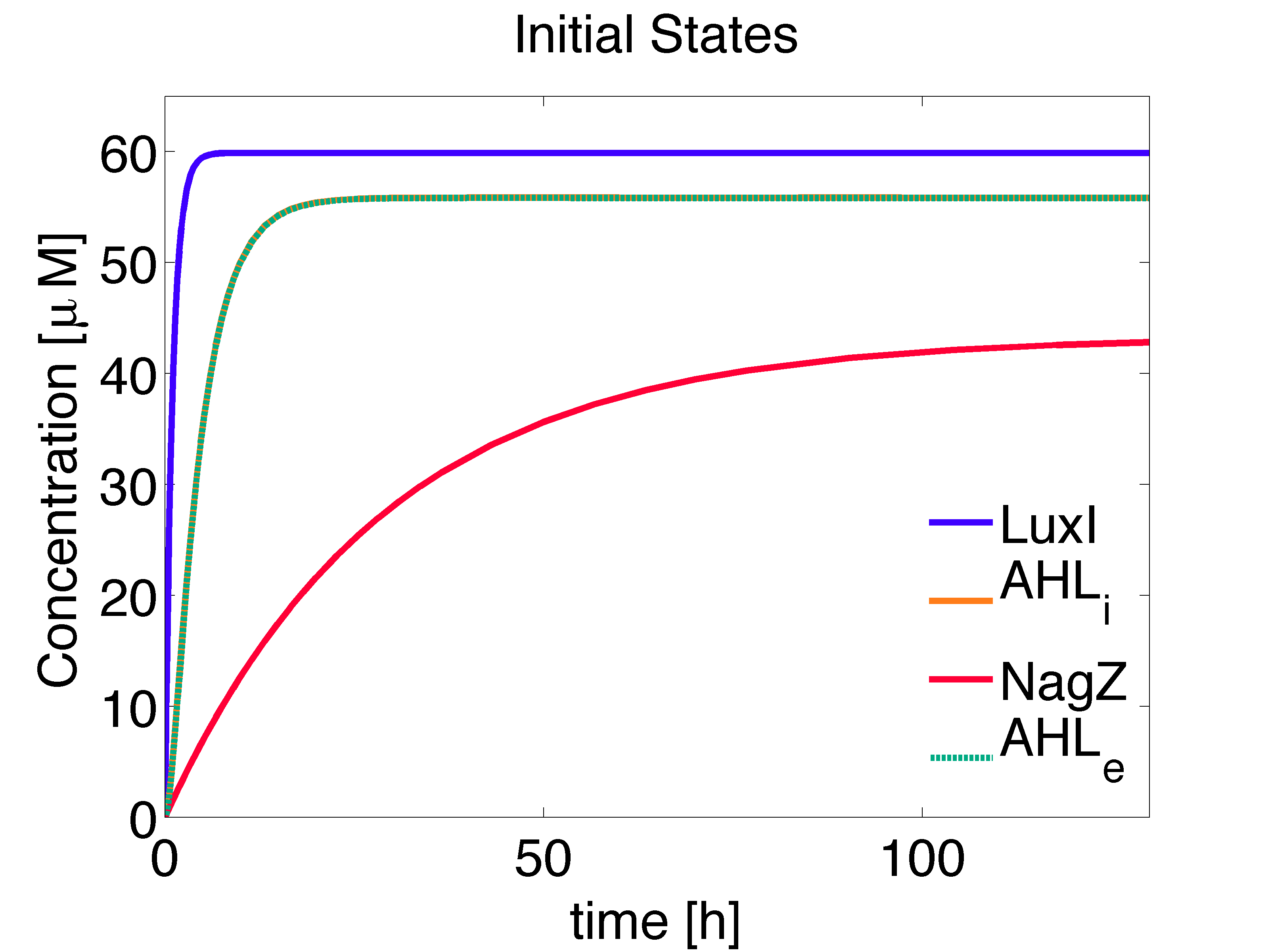
The mine colonies produce three molecules, NagZ, LuxI and AHL, that are important in our bio-game. The proteins NagZ and LuxI are constitutively produced, whereas the synthesis of AHL is dependent on LuxI. Moreover, degradation of these proteins is modeled as linear degradation. We have also taken into account the contribution of the dynamic evolution of AHL due to diffusion toward/from the medium, distinguishing between intracellular and extracellular concentrations (Garcia-Ojalvo et. al., 2004.)
The ODEs for the states involved in the mine cells are given below:
\begin{align} \frac{d[LuxI]}{dt} =\alpha_{LuxI} - d_{LuxI} \cdot [LuxI]\\ \end{align}
\begin{align} \frac{d[NagZ]}{dt} =\alpha_{NagZ} - d_{NagZ} \cdot [NagZ]\\ \end{align}
\begin{align} \frac{d[AHL,i]}{dt}= \alpha_{AHL} \cdot [LuxI]-d_{AHL,i} \cdot [AHL,i] - \eta \cdot \left([AHL,i]-[AHL,e]\right)\\ \end{align}
\begin{align} \frac{d[AHL,e]}{dt}= -d_{AHL,e} \cdot [AHL,e] + \eta_{ext} \sum_{j=1}^{n} \left([AHL,j]-[AHL,e]\right)\\ \end{align}
System of differential equations for a sender cells, where $\eta = \sigma \cdot A/V_{c}$ measures the diffusion across the cell membrane, with $\sigma$ representing the membrane permeability, $A$ its surface area and $V_{c}$ the cell volume, and $\eta_{ext} = \delta/V_{ext}$ with $V_{ext}$ being the total extracellular volume.
The concentration of the species at steady state can be solved analytically, obtaining solutions consistent with the numerical solution (Fig. 1):
\begin{align} [LuxI]_{ss} =\frac{\alpha_{LuxI}} {d_{LuxI}} \approx 59.8802 \mu M \end{align}
\begin{align} [NagZ]_{ss} =\frac{\alpha_{NagZ}} {d_{NagZ}} \approx 43.2809 \mu M \end{align}
\begin{align} [AHL,i]_{ss}= \frac{\alpha_{AHL} \cdot [LuxI]_{ss}} {d_{AHL,i} + \eta \cdot \left(1-\frac{\eta_{ext}}{d_{AHL,e}+\eta_{ext}}\right)} \approx 55.8492 \mu M \end{align}
\begin{align} [AHL,e]_{ss}= \frac{ \eta_{ext} \cdot [AHL,i]_{ss}}{d_{AHL,e} + \eta_{ext} } \approx 55.8291 \mu M \end{align}
Initial state for the Receiver Cells
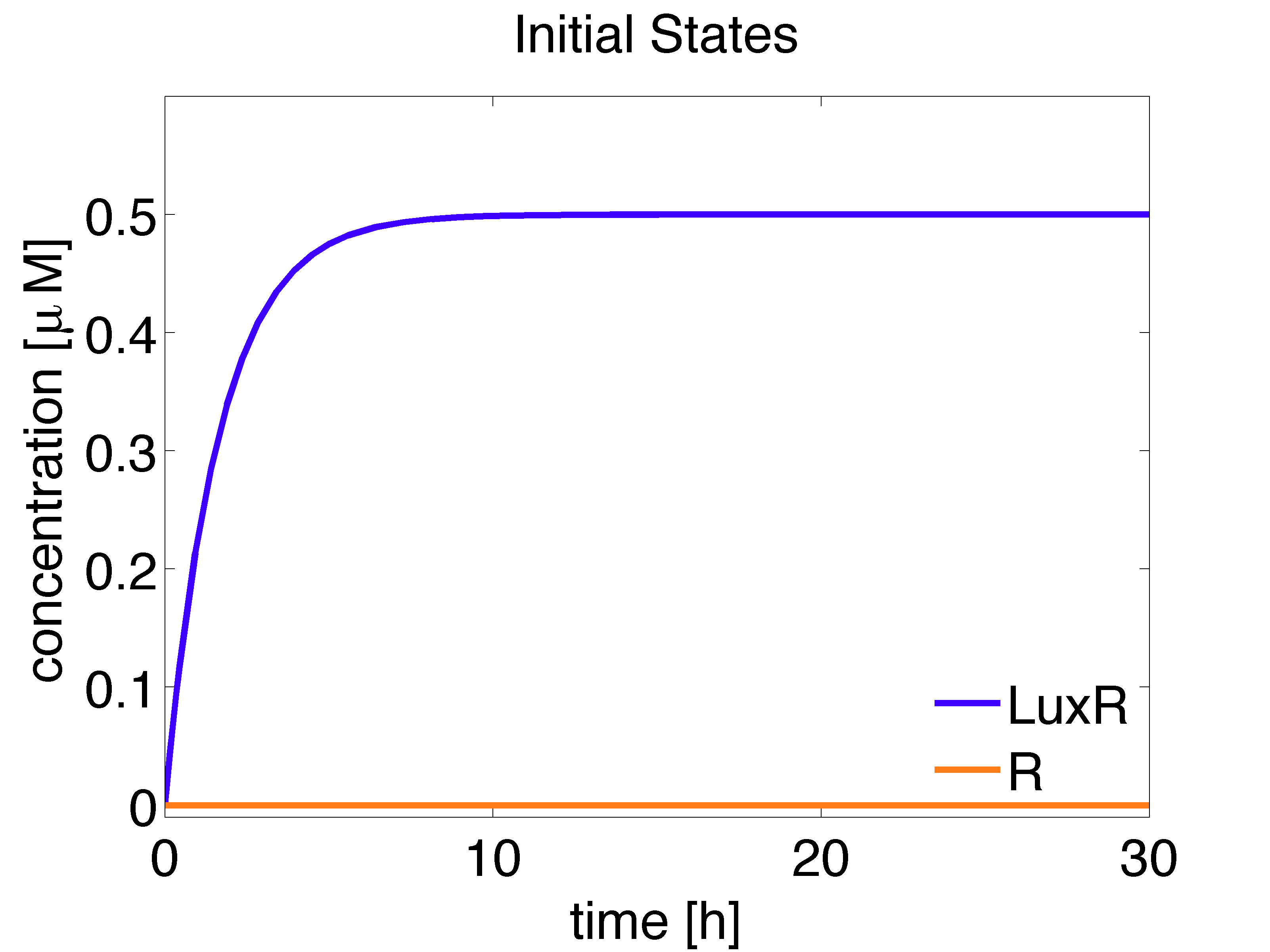
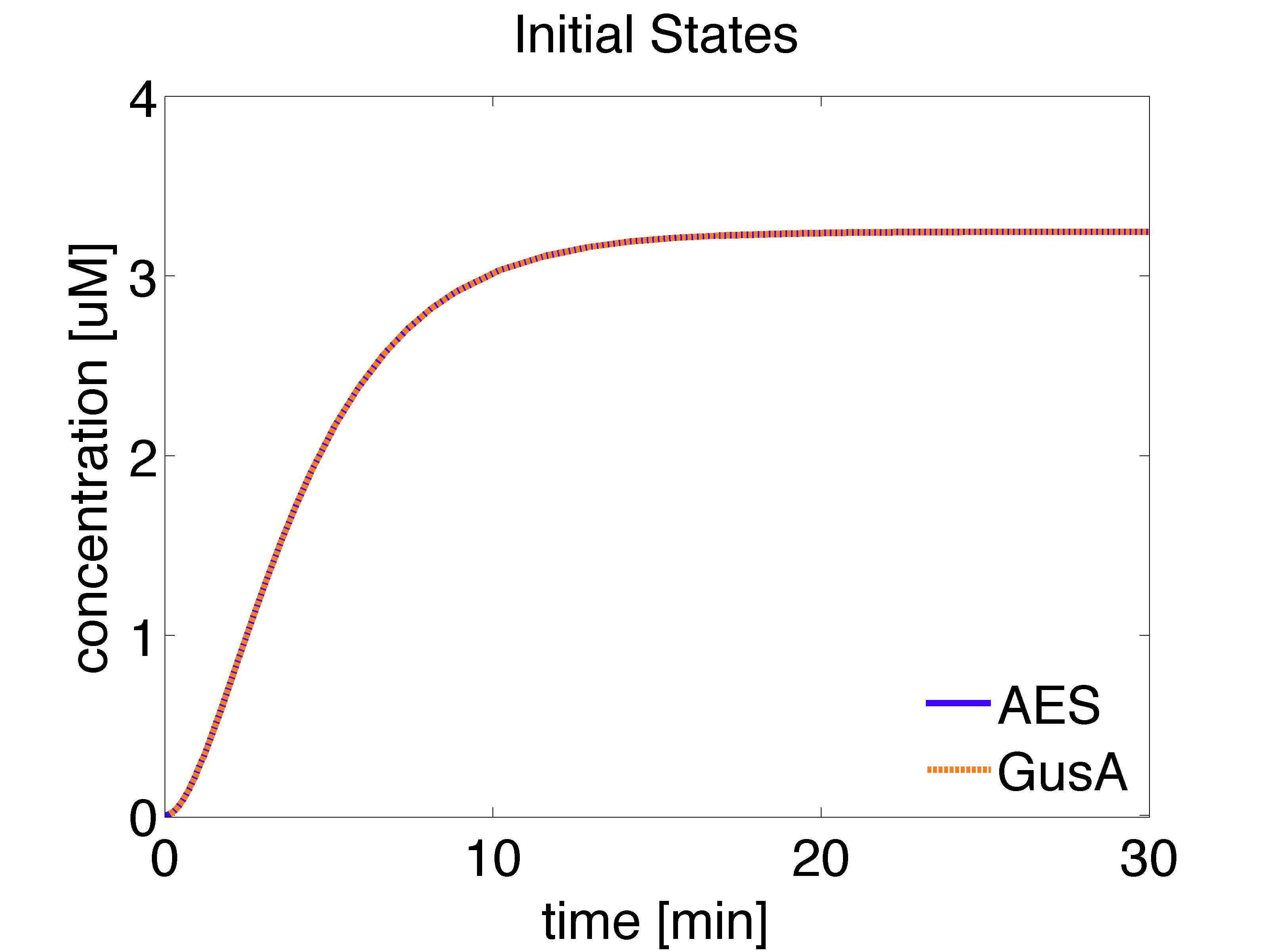
Receiver cells are responsible of processing the AHL signal and generating a visible distinguishable colour outputs. To do so, we introduce a enzymatic reporter system using the hydrolases GusA and AES. Proteins are expressed under the control of PLuxR promoters which are activated by LuxR/AHL complex (denoted as r). We used the wild type promoter to drive expression of GusA and a promoter mutant, less sensitive towards LuxR/AHL complex, for AES.
The ODEs for the states involved in the receiver cells are given below:
\begin{align} \frac{d[LuxR]}{dt}=\alpha_{[LuxR]} - d_{LuxR} [LuxR] \\ \end{align}
\begin{align} \frac{d[R]}{dt}=\rho_{LuxR} \cdot [LuxR ]^2 \cdot [AHL]^2 - d_{R} [R]\\ \end{align}
\begin{align} \frac{d[AHL,i]}{dt}= \eta \cdot \left([AHL,e]-[AHL,i]\right) -d_{AHL,i} \cdot [AHL,i]\\ \end{align}
\begin{align} \frac{d[AHL,e]}{dt}= -d_{AHL,e} \cdot [AHL,e] + \eta_{ext} \sum_{j=1}^{n} \left([AHL,j]-[AHL,e]\right)\\ \end{align}
\begin{align}
\frac{d[GusA]}{dt}=\alpha_{GusA} \cdot k_{leaky} \cdot [LuxR]+ \frac{\alpha_{GusA}(\frac{[R]}{K_{R_1}})^{n}}{1+(\frac{[R]}{K_{R_1}})^{n}} - d_{GusA}\cdot [GusA]\\
\end{align}
\begin{align} \frac{d[AES]}{dt}=\alpha_{AES} \cdot k_{leaky} \cdot [LuxR] + \frac{\alpha_{AES}(\frac{[R]}{K_{R_2}})^{n}}{1+(\frac{[R]}{K_{R_2}})^{n}} - d_{AES}\cdot [AES]\\ \end{align}
It should be noted that the receiver cells grow in the absence of AHL in the medium and this affects the concentrations of intracellular species whose production depends on AHL.
The analytical solution for the steady state is given below:
\begin{align} [LuxR]_{ss}= \frac{\alpha_{LuxR}}{d_{LuxR}} \approx 0.5 \mu M \end{align}
\begin{align} [GusA]_{ss}= \frac{\alpha_{GusA} \cdot k_{leaky} \cdot [LuxR]_{ss}}{d_{GusA}} \approx 3.2461 \mu M \end{align}
\begin{align} [AES]_{ss}= \frac{\alpha_{AES} \cdot k_{leaky} \cdot [LuxR]_{ss}}{d_{AES}} \approx 3.2461 \mu M \end{align}
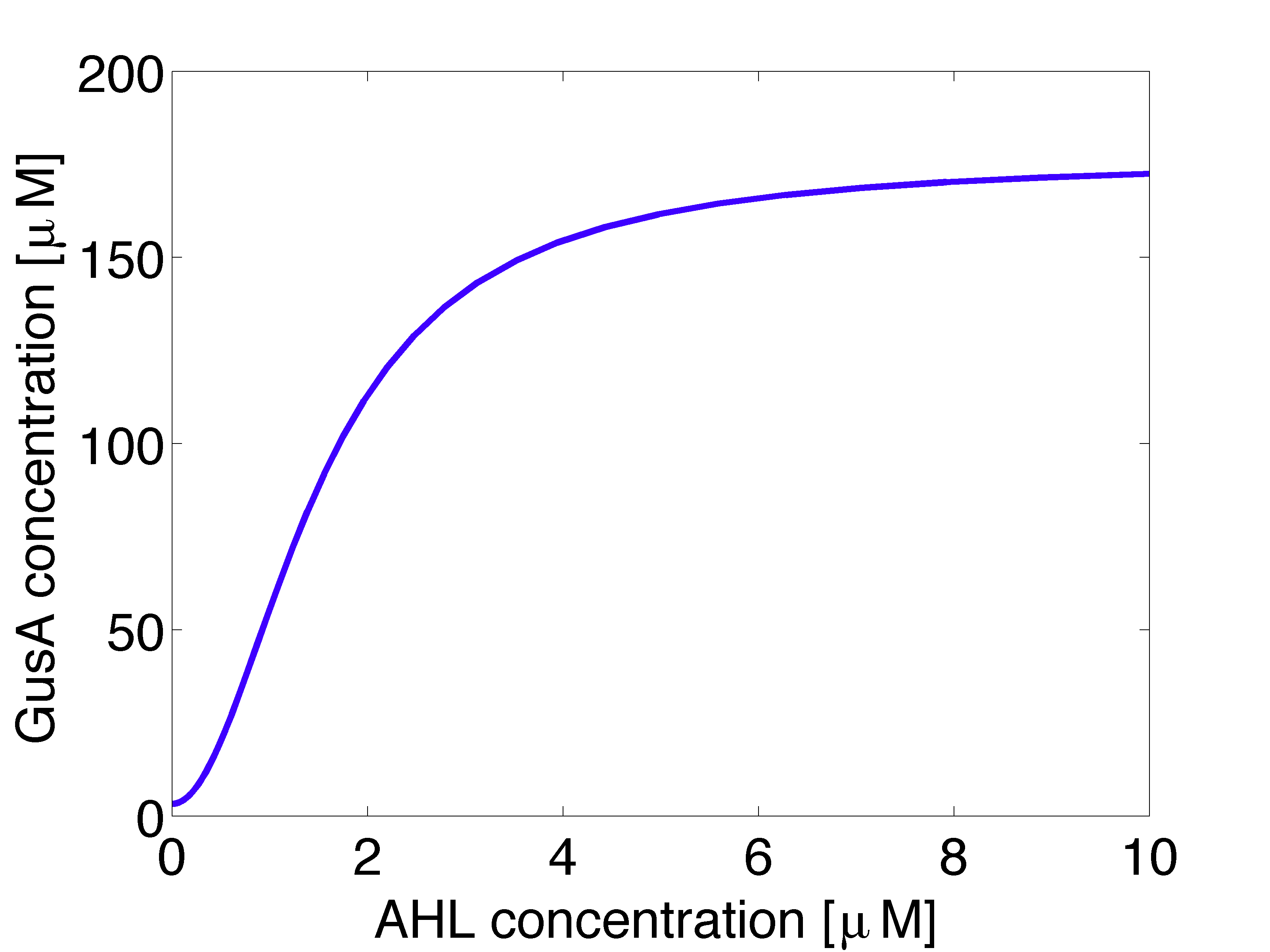
Output System
GusA expression should be triggered by one mine in the vicinity. In figure 4 is shown the GusA steady state concentration as function of AHL as input. We simulated the system of equations for 5000 minutes.
 "
"