Team:Paris Saclay/Notebook/August/29
From 2013.igem.org
(→7 - Electrophoresis to check the digestion of BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI/PstI and plasmids already digested by SpeI and after digested by PstI) |
(→3 - Digestion of BBa_K1155000, BBa_K1155003, BBa_K1155007 by Xbal/PstI) |
||
| Line 78: | Line 78: | ||
** H2O : 9µL | ** H2O : 9µL | ||
| - | We | + | We incubatethe digestion for 30 minutes at 37°C. |
===='''4 - Electrophoresis to check the digestion of BBa_K1155000 by PstI/SpeI, BBa_K1155003, BBa_K1155007 by Xbal/PstI'''==== | ===='''4 - Electrophoresis to check the digestion of BBa_K1155000 by PstI/SpeI, BBa_K1155003, BBa_K1155007 by Xbal/PstI'''==== | ||
Revision as of 15:49, 4 October 2013
Notebook : August 29
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000 and BBa_K1155004, BBa_K1155005, BBa_K1155006
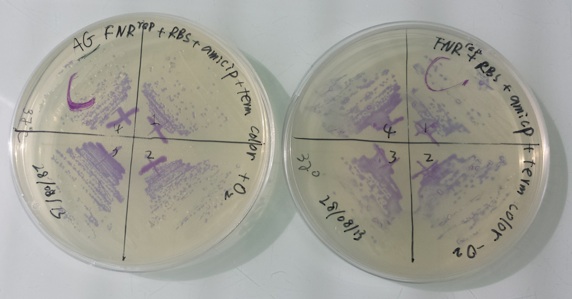
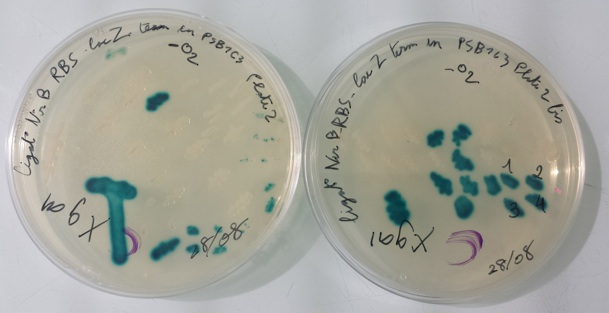
1 - Purification of colony transformed with ligation : NirB with RBS-LacZ-Term in PSB1C3, Pfnr with RBS-Amil CP-Term in PSB1C3 by streaking in aerobic or anaerobic conditions
XiaoJing
|
Purification of 08/28/13 didn't work. We have blue colonies for Pfnr with RBS-Amil CP-Term in pSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for NirB with RBS-LacZ-Term in pSB1C3 in anaerobic conditions. We will streak these colonies again. |
We streak :
- Pfnr with RBS-Amil CP-Term in pSB1C3 with O2 at 37°C
- Pfnr with RBS-Amil CP-Term in pSB1C3 without O2 at 37°C
- Pfnr with RBS-Amil CP-Term in pSB1C3 with O2 at 30°C
- Pfnr with RBS-Amil CP-Term in pSB1C3 without O2 at 30°C
- NirB with RBS-LacZ-Term in pSB1C3 with O2 with Xgal at 37°C
- NirB with RBS-LacZ-Term in pSB1C3 without O2 with Xgal at 37°C
We also purify Pfnr with RBS-Amil CP-Term in pSB1C3 in liquid culture at 37°C using :
- Pfnr with RBS-Amil CP-Term in pSB1C3 in aerobic conditions :
- LB : 10 mL
- Clone : 1 and 2
- Pfnr with RBS-Amil CP-Term in pSB1C3 in anaerobic conditions :
- LB : 50mL
- Clone : 1 and 2
2 - Colony PCR of Pfnr with RBS_AmilCP-Term in DH5α
XiaoJing
We mix our colonies in 20µL of H2O.
Used quantities :
- DNA : 2µL
- Mix : (it was divided in 4 tubes for 4 different colonies for each assembly with 23µL of mix in each tube. We do it twice.)
- Oligo 43 : 14µL
- Oligo 44 : 14µL
- dNTP : 14µL
- Buffer Dream Taq : 69µL
- Dream Taq : 5.5µL
- H2O : 577µL
3 - Digestion of BBa_K1155000, BBa_K1155003, BBa_K1155007 by Xbal/PstI
XiaoJing
We used clone 9 and 12 for BBa_K1155003, clone 10, 11 and 15 for BBa_K1155007
Used quantities :
- BBa_K1155003, BBa_K1155007
- DNA : 14µL
- Buffer FD : 2µL
- XbaI FD : 2µL
- PstI FD : 2µL
- BBa_K1155000 :
- DNA : 5µL
- Buffer FD : 2µL
- SpeI FD : 2µL
- PstI FD : 2µL
- H2O : 9µL
We incubatethe digestion for 30 minutes at 37°C.
4 - Electrophoresis to check the digestion of BBa_K1155000 by PstI/SpeI, BBa_K1155003, BBa_K1155007 by Xbal/PstI
XiaoJing
| [[]] |
|
Expected sizes :
- RBS-LacZ-Term : 3500bp
- RBS-Amil CP-Term :
| [[]] |
|
Expected sizes :
- Pfnr :
|
We obtain fragments at the right size. We will purify them. |
5 - Gel purification of the digestion of BBa_K1155000 by PstI/SpeI, BBa_K1155003, BBa_K1155007 by Xbal/PstI
XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- Pfnr : 26.2ng/µL
6 - Electrophoresis to check the gel purification of the digestion of BBa_K1155000 by PstI/SpeI, BBa_K1155003, BBa_K1155007 by Xbal/PstI
Damir
| [[]] |
|
Expected sizes :
- RBS-LacZ-Term : 3500bp
- RBS-Amil CP-Term :
|
We obtain fragments at the right size for RBS-LacZ-Term. We will ligate it. |
7 - Electrophoresis to check the digestion of BBa_K1155004, BBa_K1155005, BBa_K1155006 by SpeI/PstI and plasmids already digested by SpeI and after digested by PstI
XiaoJing
| [[]] |
|
Expected sizes :
- NarK, NarG, NirB : 200bp
|
We obtain fragments at the right size for NarK. We will ligate it |
Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in pSB3K3
1 - Digestion of BBa_J04450 by EcoRI/PstI
Anaïs
Used quantities :
- Buffer FD: 2µL
- H2O : 5µL
- DNA : 9µL
- EcoRI FD : 1µL
- PstI FD : 1µL
We let the digestion at 37°C during 10 minutes.
2 - Electrophoresis to check the digestion of BBa_J04450 by EcoRI/PstI
XiaoJing
| [[]] |
|
Expected sizes :
- pSB3K3 : 2750bp
|
We obtain fragments at the right size. We will ligate it. |
3 - Gel purification of the digestion of BBa_J04450 by PstI/SpeI
XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
Nanodrop :
- Pfnr : 7.2ng/µL
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FRN and BphR2 proteins
1 - Colony PCR of FNR, RBS-FNR and RBS-BphR2 in DH5α
XiaoJing
|
Transformation of 08/28/13 works. We will do a Colony PCR. |
We mix our colonies in 20µL of H2O.
Used quantities :
- DNA : 2µL
- Mix : (it was divided in 8 tubes for 8 different colonies for each assembly with 23µL of mix in each tube. We do it twice.)
- Oligo 43 : 27.5µL
- Oligo 44 : 27.5µL
- dNTP : 27.5µL
- Buffer Dream Taq : 137.5µL
- Dream Taq : 11µL
- H2O : 1144µL
| Previous day | Back to calendar | Next day |
 "
"