Team:Bielefeld-Germany/Project/Riboflavine
From 2013.igem.org
| Line 183: | Line 183: | ||
==Results== | ==Results== | ||
| - | + | ===Confirming RibE=== | |
| - | + | The Protein RibE belongs to the riboflavin synthesis pathway of '''Shewanella oneidensis | |
| + | ''' | ||
*Riboflavin | *Riboflavin | ||
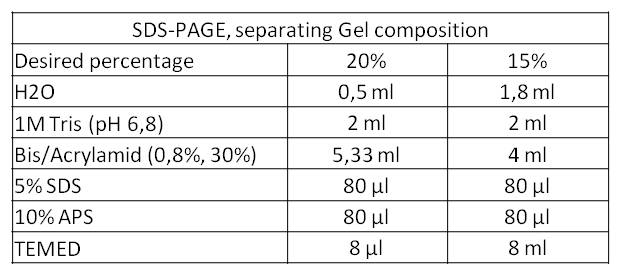
**We performed a third, fourth and fifth SDS-PAGE. This time we used a 20% separating gel. We aimed at the separation of proteins with a low molecular weight because the rib-cluster consists of four genes that code for five proteins of which four lay in the range of 16 kDa till 23 kDa. | **We performed a third, fourth and fifth SDS-PAGE. This time we used a 20% separating gel. We aimed at the separation of proteins with a low molecular weight because the rib-cluster consists of four genes that code for five proteins of which four lay in the range of 16 kDa till 23 kDa. | ||
Revision as of 22:24, 4 October 2013
Riboflavin
Overview

Tubes from left to right:
- Tube 1: A 72-hour culture of E. coli KRX [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1172306 BBa_K1172306]
- Tube 2: A 72-hour culture of E. coli KRX [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1172306 BBa_K1172306], supernatant
- Tube 3: A 72-hour culture of E. coli KRX [http://parts.igem.org/wiki/index.php?title=Part:BBa_K1172306 BBa_K1172306], cell lysate
- Tube 4: A 72-hour culture of a wild type E. coli KRX, supernatant
- Tube 5: A 72-hour culture of a wild type E. coli KRX, cell lysate
[http://de.wikipedia.org/wiki/Riboflavin Riboflavin], or Vitamin B2 is a redox-active substance that plays an
essential role in living cells. Secreted into the medium, it can be
effectively used by some bacteria for electron transfer. Presence of riboflavin in anaerobic cultures leads to higher current flow in a
microbial fuel cell, which made riboflavin overproduction a suitable target for optimisation of our MFC.
We have shown that cloning of the riboflavin cluster from a metal-reducing bacterium Shewanella oneidensis MR-1 in E. coli is sufficient to achieve
significant riboflavin overproduction detectable both in supernatant and in cells.
Theory
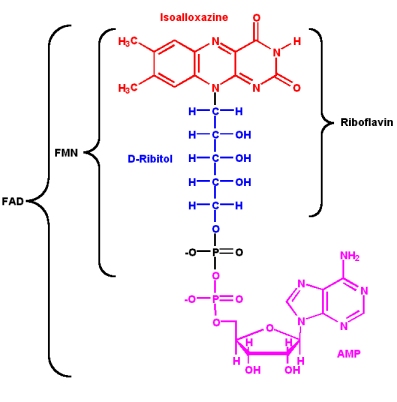
Since its discovery in 1879 and its first structural characterization in the 1930’s, a lot of properties of riboflavin were elucidated. This substance is a precursor of FMN (flavin mononucleotide) and FAD (flavin adenine dinucleotide), which play an essential role as cofactors in many oxidative processes.
The modern name riboflavin, also named lactoflavin, is composed of two parts: «ribo» indicating the presence of the sugar alcohol ribitol, and «flavin» meaning «yellow»; to accentuate the yellow coloring of the oxidized molecule. Chemically this substance consists of two functional subunits, an already mentioned short-chain ribitol and a tricyclic heterosubstituted isoalloxazine ring.
The latter, also known as a riboflavin ring, exists in three redox states and is responsible for the diverse chemical activity of riboflavin. A fully oxidized quinone, a one-electron semiquinone and a fully reduced hydroquinone states are the three stages of riboflavin oxidation. In an aqueous solution, the quinone (fully oxidized) form of riboflavin has a typical yellow coloring. It becomes red in a semi-reduced anionic or blue in a neutral form and is colorless when fully reduced.
All these forms are present in different proportions in a living cell, making previous oxidation a necessary step if riboflavin analysis is to be conducted. Flavins have a typical yellow-green fluorescence in the UV light. The peaks of absorbance occur at 223, 266, 373 and 445 nm. The maximum fluorescence emission of the neutral solution is at 535 nm [Charles A. Abbas et al., [http://mmbr.asm.org/content/75/2/321.full#ref-292 2011]]. These fluorimetric properties are widely used in the analysis of riboflavin.
Due to its structure, which allows a transfer of two electrons from hydrogen and hydrid ions, riboflavin can be imagined as a potential electron shuttle. It was previously known, that the electron transfer from the outer membrane-associated proteins to an inorganic electron acceptor is the main limiting growth factor for Fe(III)-reducing prokaryotes, so a few mechanisms that show how this process can be enhanced have been discovered. One of them was a secretion of water-soluble redox mediators. It was proven, that secretion of riboflavin and FMN enhances the rate of insoluble mineral oxides reduction. Indeed, Shewanella oneidensis, a facultative Fe-III respiring bacterium uses secreted riboflavin as its electron transmitter [Harald von Canstein et al., [http://aem.asm.org/content/74/3/615.full 2008]]. Considering this acknowledgement, we decided to overproduce riboflavin in E. coli to improve its efficiency in our MFC.
First of all, we have searched for a suitable microorganism, which has an active riboflavin cluster with known coding sequences. We have already used the remarkable versatility of Shewanella in order to clone the anaerobic respiratory chain (mtrCAB cluster), so now we were able to skip some initial steps, like the whole genome DNA isolation and advanced rapidly to more specific steps.
Before planning the cloning strategy, we checked [http://parts.igem.org/Main_Page the Parts registry] for any parts we could use or enhance. A [http://parts.igem.org/Part:BBa_K769203 ribC part] was listed, but it was neither submitted nor available.
We also had a close look on the [http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/riboflavin2.html riboflavin biosynthesis pathway]. This process is well studied and a lot of appropriate literature is available. There are three types of riboflavin overproducers used in industry: yeast (Candida famate), fungi (Ashbya gossypii), and bacteria (Bacillus subtilis) [Overview, Seong Han Lim et al., [http://www.bbe.or.kr/storage/journal/BBE/6_2/6657/articlefile/article.pdf 2001]], but a prevailing majority of microorganisms also synthesize riboflavins in low concentrations. In E. coli, for instance, genes are scattered through the whole genome and riboflavin is produced constitutively.
The biosynthesis of riboflavin starts with ribulose-5-phosphate and GTP, converted to formate and DHBP (L-3,4-dihydroxybutan-2-one 4-phosphate). The final stage involves forming of the third isoalloxazine ring by an exchange of a 4-carbon part, catalysed by riboflavin synthetase (EC 2.5.1.9). We assumed, that the operon in Shewanella could be similar to a well-studied [http://www.ncbi.nlm.nih.gov/pubmed/8159171 rib-operon] of Bacillus subtilis. The operon is transcribed as a one polycistronic RNA, making a single promoter sufficient. Introduction of multiple copies of a ribA gene (coding for GTP cyclohydrolase II (EC 3.5.4.25)), comprised in the rib-operon, results in riboflavin overproduction in B. subtilis [Hohmann H.P.,Stahmann K.P. 2010. Biotechnology of riboflavin production, p. 115–139.], so we predicted a notable riboflavin synthesis gain following a successful introduction of a multiple-copy plasmid harbouring the rib-operon under an active promoter.
Genetic Approach
Riboflavin Cluster

Below we shortly describe each functional member of this cluster.
- Gene: RibD http://www.ncbi.nlm.nih.gov/gene/1171145 Sequence, 1145 bp
- Protein: bifunctional diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-amino-6-(5-phosphoribosylamino) uracil reductase RibD
- Enzyme: (EC: 3.5.4.26)
- Molecular weight: 41,257 kDa
- Gene: SO_3468 http://www.ncbi.nlm.nih.gov/gene/1171144 Sequence, 656 bp
- Protein: Riboflavin synthase alpha subunit RibC-like protein
- Enzyme: EC 2.5.1.9
- Molecular weight: 23,483 kDa
- Gene: RibBA → ribA & ribB http://www.ncbi.nlm.nih.gov/gene/1171143 Sequence, 1103 bp
- ribA
- Protein: GTP cyclohydrolase-2
- Enzyme: EC 3.5.4.25
- Molekular weight: 22,852 kDa
- ribB
- Protein: 3,4-dihydroxy-2-butanone-4-phosphate synthase
- Enzyme: EC 3.5.4.25
- Molecular weight: 22,956 kDa
- Gene: ribE http://www.ncbi.nlm.nih.gov/gene/1171142 Sequence, 476 bp
- Protein: 6,7-dimethyl-8-ribityllumazine synthase (aka: riboflavin synthase beta subunit RibE)
- Enzyme: EC 2.5.1.78
- Molecular Weight: 16,689 kDa
Figure 5 illustrates the initial position we established first: Isolating of the rib-gene cluster from the genome of Shewanella oneidensis and clone it into pSB1C3.

Supplementary Genes
We also separately amplified some other genes that theoretically could be important for riboflavin synthesis or regulation.
- Gene: ribC http://www.ncbi.nlm.nih.gov/gene/1170021 Sequence, 621 bp
- Protein: 6,7-dimethyl-8-ribityllumazine synthase alpha subunit RibC, analogous to EC 2.5.1.9
- Molecular Weight: 22,110 Da
- Gene: nusB (full name: N utilization substance protein B homolog), http://www.ncbi.nlm.nih.gov/gene/1171141 Sequence, 403 bp
- Protein: transcription antitermination factor
- Molecular weight: 14,740 Da
- Gene: norM, http://www.ncbi.nlm.nih.gov/gene/1170020 Sequence, 1380 bp
- Protein: probable multidrug eflux protein NorM
- Molecular weight: 50,490 Da

Results
Confirming RibE
The Protein RibE belongs to the riboflavin synthesis pathway of Shewanella oneidensis
- Riboflavin
- We performed a third, fourth and fifth SDS-PAGE. This time we used a 20% separating gel. We aimed at the separation of proteins with a low molecular weight because the rib-cluster consists of four genes that code for five proteins of which four lay in the range of 16 kDa till 23 kDa.
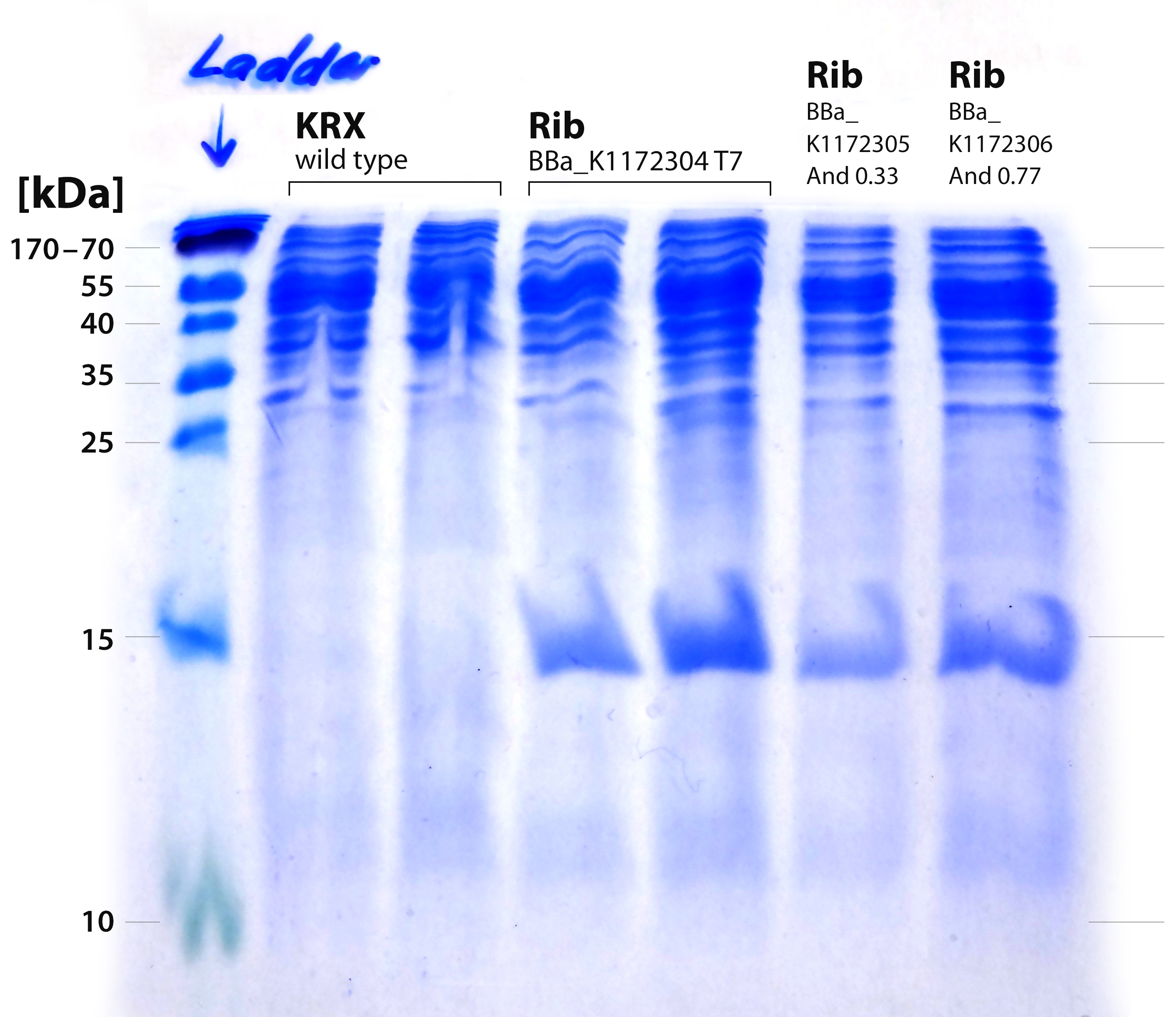
- It is obvious that a protein with a molecular weight of approximately 15 kDa was overexpressed by our riboflavin strains (see figure X). We cut out the band of the T7-induced strain and handed it out to Vera. She is allowed to perform MALDI´s and was friendly enough to help us out.
- In the middle of the week, it became clear that the M9 medium we had used the last one and a half week had a negative effect on riboflavin production in our recombinant strain. While working on the MFC subproject Matthias produced another M9 medium with different trace solutions (e.g.: a different iron-salt). In this medium, our riboflavin strain produced high amounts of riboflavin that were clearly visible as yellow colour.
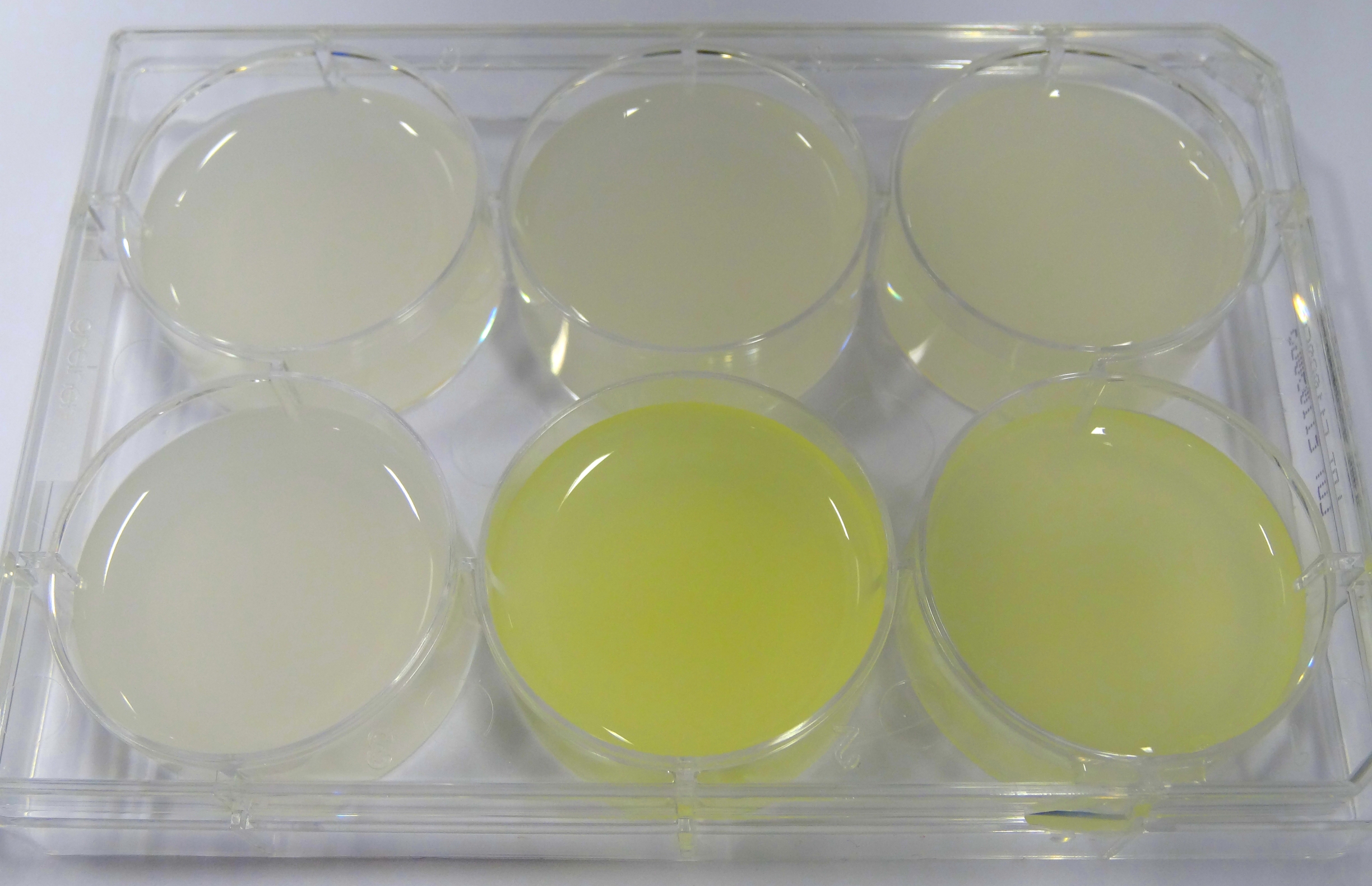
Figure X: Comparison of colour change in riboflavin strains. Left: e. coli KRX wildtype; Middle top: Ribo-strain with strong Anderson-promoter (And77) in wrong M9-medium; Middle below: same strain in good M9; Right top: Ribo-strain with medium Anderson-promoter (And33) in wrong M9; Right below: same strain in good M9
We are calling this good M9 medium, which is favorable for riboflavin production: M9-D5. - We used a culture of E.coli KRX grown in M9-D5, which carried pSB1C3 with the rib-cluster and a strong Anderson Promoter (And 77) with strong RBS (<bbpart>BBa_K1172306</bbpart>) to qualitative proof the appearance of riboflavin in supernatant.
- In the middle of the week, it became clear that the M9 medium we had used the last one and a half week had a negative effect on riboflavin production in our recombinant strain. While working on the MFC subproject Matthias produced another M9 medium with different trace solutions (e.g.: a different iron-salt). In this medium, our riboflavin strain produced high amounts of riboflavin that were clearly visible as yellow colour.
- We measured the absorbance at 446 nm of the M9-D5 supernatant in which E. coli KRX with BBa_K1172306 had grown for 72 hours and cell disruption samples of these cultures against wild type supernatant and cell disruptions who had also grown for 72 hours. We also measured a dilution series of riboflavin in known concentrations.
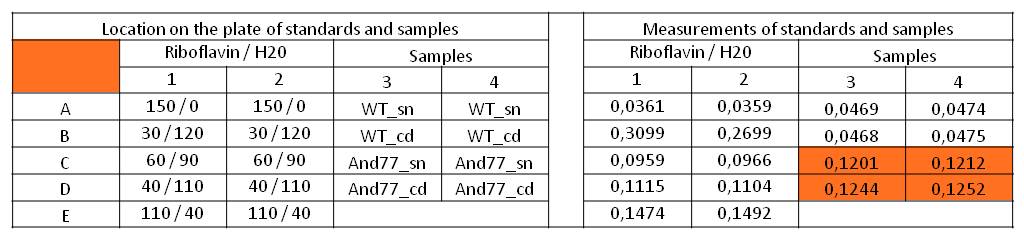
Table X: Pipetting scheme and measurement results of riboflavin standards and cell samples for absorbance measurement at 446 nm in the [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]. WT = wild type, And77 = Coli equipped with BBa_K1172306, sn = supernatant, cd = cell disruption.
- First of all, this clearly showed that the amount of riboflavin produced by our riboflavin production strain was significantly higher than in the wild type. Furthermore, on the basis of the known riboflavin concentrations we were able to generate a calibration line:
- Y = 4839.6*X – 0,0462
- This allowed us to evaluate the amount of riboflavin in the supernatant and the cell disruption samples.
- Supernatant: 5773.3 µg / L
- Cell disruption: 6112.63 µg /L
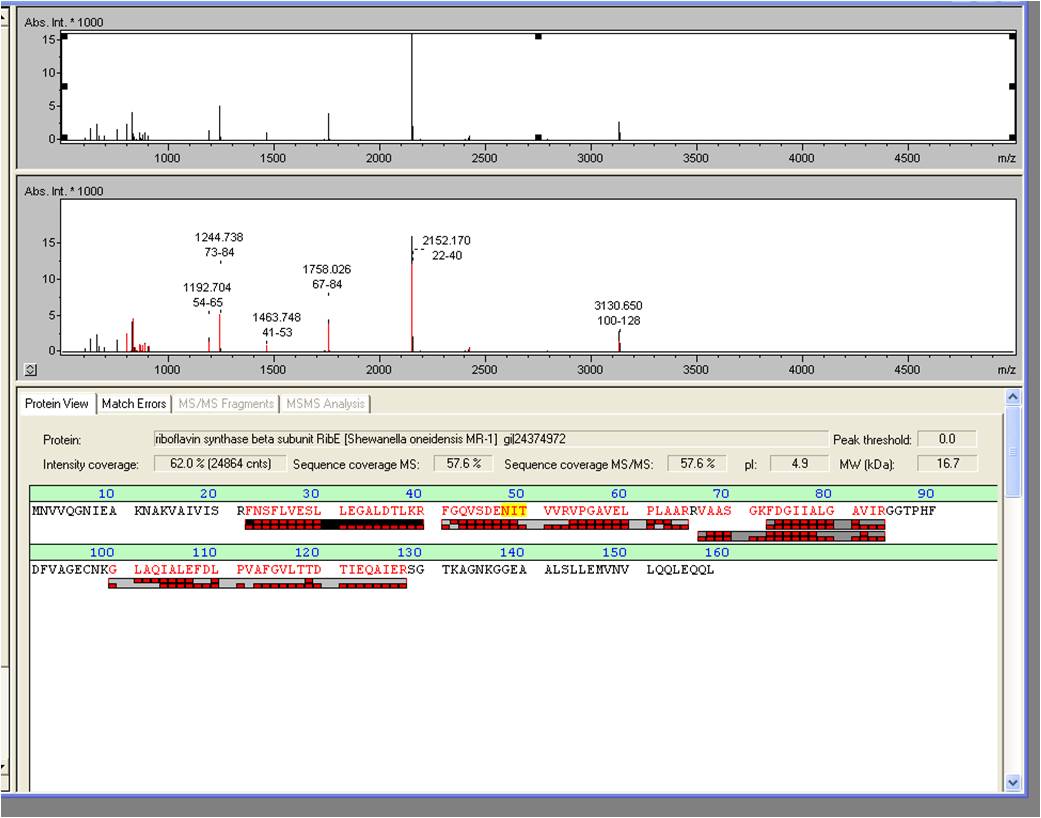
- We received the data from the MALDI-TOF and the subsequent results where positive: The overexpressed protein, had formed a band on a SDS-PAGE at about 15 kDa. We hoped it would turn out to be RibE, with a molecular weight of 16,7 kDa.
- The results show that it is indeed “riboflavin synthase beta subunit ribE” from ``Shewanella oneidensis`` MR-1. The mascot score was a slick 906. We want to thank Vera Ortseifen for the execution of our measurements.
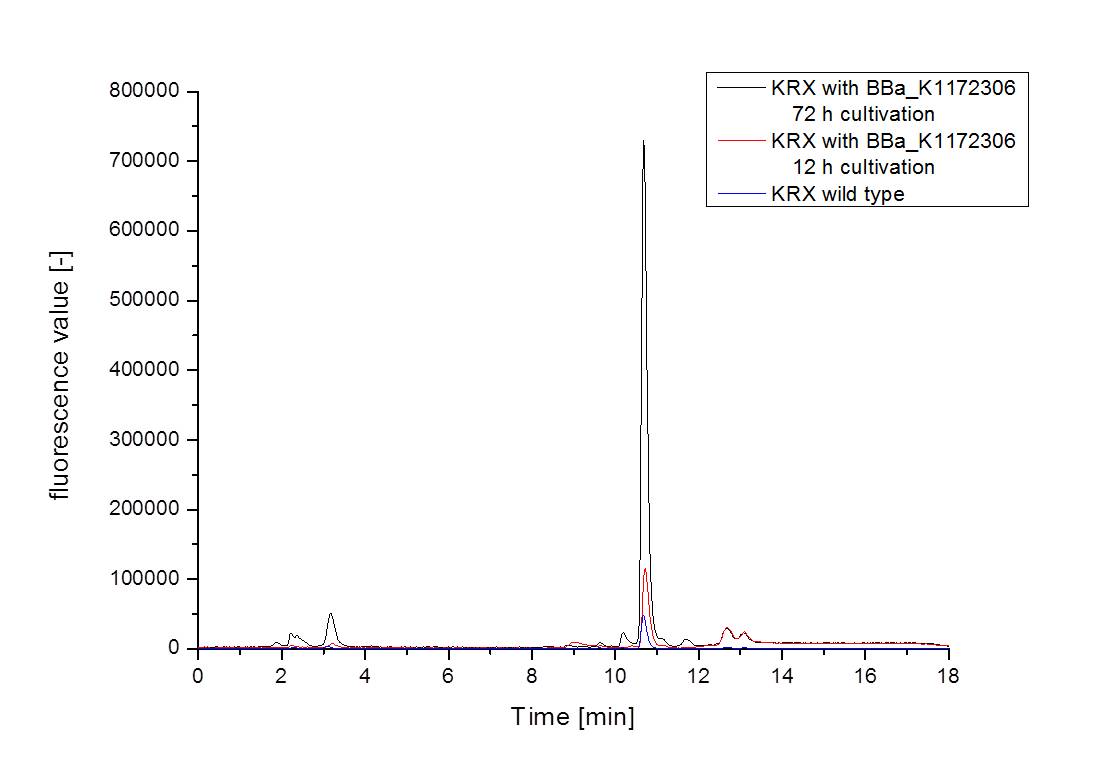
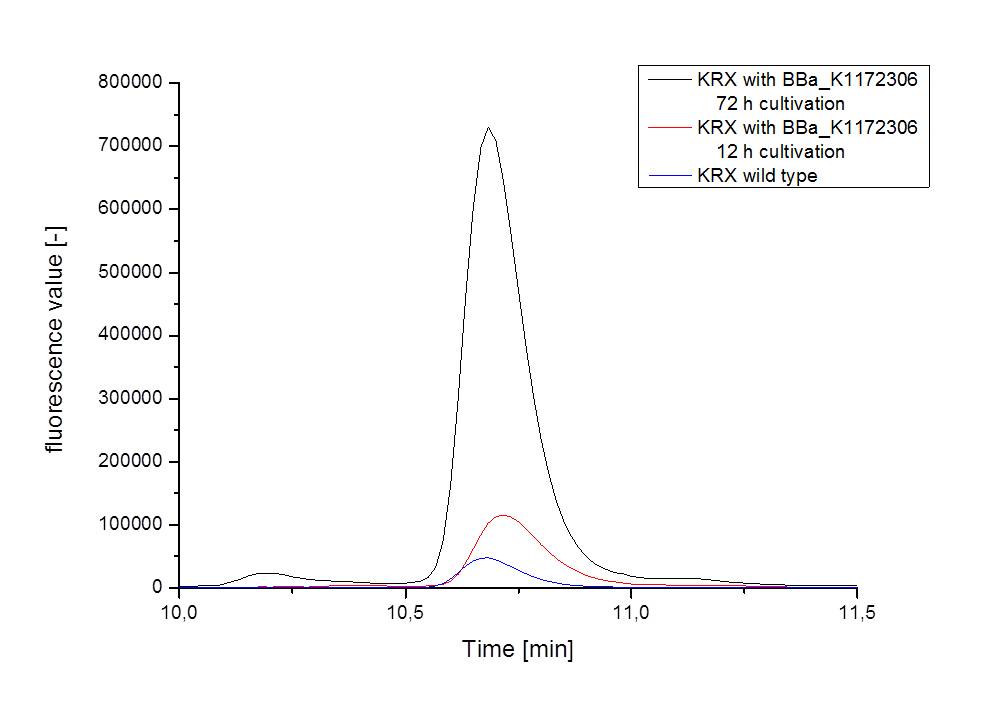
- For further quantitative analysis of riboflavin in culture supernatants we measured fluorescence with the [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader] (The week before we had already measured absorbance). Riboflavine absorbes light at 440 nm with a corresponding emission at 535 nm. We again checked riboflavin of known quantities to compute a calibration line. The samples consisted of KRX wild type bacteria (grown for 72 hours) and KRX that carried the rib-cluster with strong Anderson promoter / strong RBS (<bbpart>BBa_K1172306</bbpart>) (grown for 72 hours and for 12 hours respectively). Of course both cultures were nurtured in the good M9-D5.
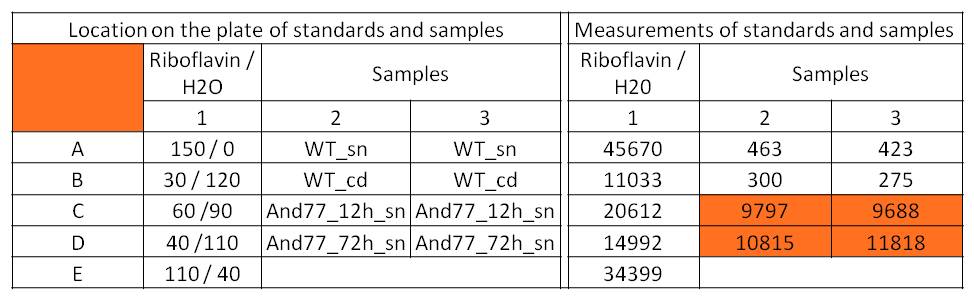
Table X: Pipetting scheme and measurement results of riboflavin standards and cell samples for fluorescence measurement, emission at 535 nm. Measured in the [http://www.tecan.com/platform/apps/product/index.asp?MenuID=1812&ID=1916&Menu=1&Item=21.2.10.1 Tecan Infinite® M200 platereader]. WT = wild type, And77 = Coli equipped with <bbpart>BBa_K1172306</bbpart>, sn = supernatant, cd = cell disruption.
- Y = 8*10^8 * X - 3193.6
- This allowed us to evaluate the amount of riboflavin in the supernatant and the cell disruption samples.
- Supernatant after 12 hours: 308.1 µg / L
- Supernatant after 72 hours: 3821.5 µg /L
- We asked the lab of Dr. Heino Büntemeyer if we could use their HPLC detector to verify riboflavin concentrations. We prepared some samples and overviewed the measurement. The HPLC-method itself was friendly carried out by Jana Heinrich. It yielded significant results and allows the quantification of riboflavins in our samples.
- Table X shows the produced amount of Riboflavin. After 72 hours of cultivation in M9-D5 the concentration of riboflavin in supernatant and cell disruption samples of ``E. coli`` KRX+<bbpart>BBa_K1172306</bbpart> was 60fold higher than in ``E. coli`` KRX wild type. Even after 12 hours, the riboflavin producing strain had generated ten times as much riboflavin as the wild type.
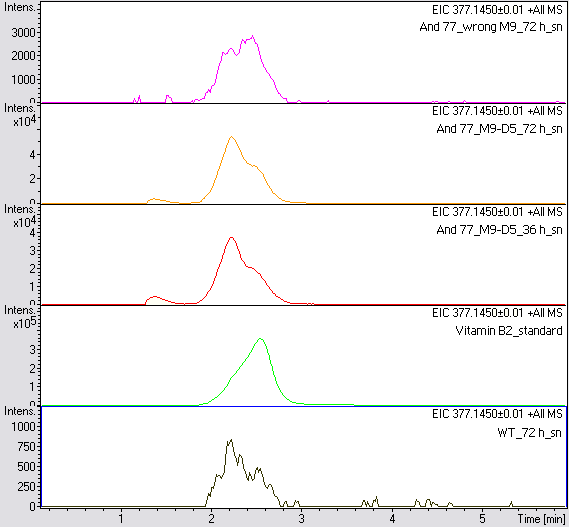
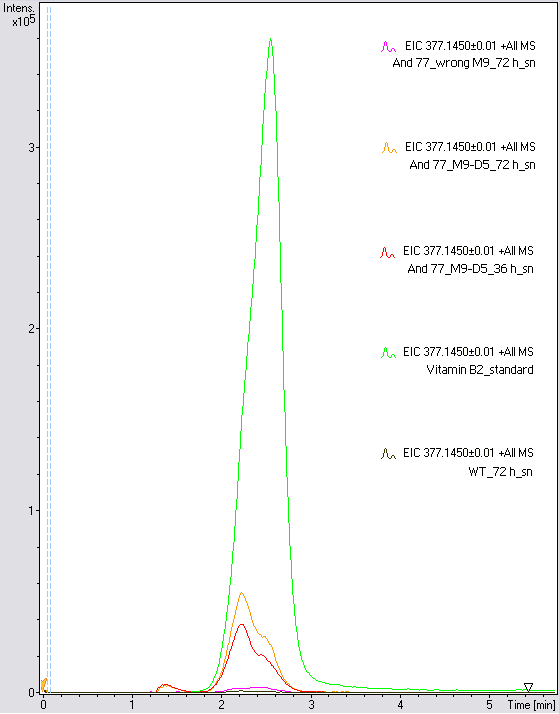
- The wrong M9 medium had been the reason for unsufficient riboflavin production. This fact rendered our first (LC/MS) measurement innocuous. We were able to organize another measurement of some of our samples with liquid chromatography-mass spectrometry (LC/MS). We prepared supernatant samples of a wild type KRX after 72 h, the <bbpart>BBa_K1172306</bbpart> strain after 36 h and 72 h in M9-D5 and after 72 h in wrong M9. In the following we put the samples in the LC-MS-system. The measurement procedure was carried out by Lena Sinkel.
- It is clear, that the <bbpart>BBa_K1172306</bbpart> carrying strains produce a much higher amount of riboflavin compared to the wild type KRX strain. The LC/MS results do not allow for a statement on how much more riboflavin our strain produces, because from this method we can´t know if the relation between the amount of vitamin B2 and the peak-area is linear. Nevertheless, it is obvious that riboflavin was overexpressed in a remarkable quantity.
It is although important to notice that the green curve in figures X represents the high concentrated riboflavin standard. A look at figure X and the relative intensities given on the Y-axis, illustrates how much more efficient KRX+<bbpart>BBa_K1172306</bbpart> in M9-D5 produces riboflavin compared to KRX+<bbpart>BBa_K1172306</bbpart> in wrong M9 and especially compared to wild type KRX.
- It is clear, that the <bbpart>BBa_K1172306</bbpart> carrying strains produce a much higher amount of riboflavin compared to the wild type KRX strain. The LC/MS results do not allow for a statement on how much more riboflavin our strain produces, because from this method we can´t know if the relation between the amount of vitamin B2 and the peak-area is linear. Nevertheless, it is obvious that riboflavin was overexpressed in a remarkable quantity.
References
C. A. Abbas and A. S. Sibirny. (2011) Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. [http://mmbr.asm.org/content/75/2/321.full#ref-292| Microbiology and Molecular Biology Reviews 75(2): 321-360].
Hohmann H. P., Stahmann K. P. (2010). Biotechnology of riboflavin production, p. 115–139. In Mander L., Liu H. W. (ed.), Comprehensive natural products. II. Chemistry and biology, vol. 7. Cofactors. Elsevier, Philadelphia, PA.
von Canstein H., Ogawa J., Shimizu S., Lloyd J. R. (2008). Secretion of flavins by Shewanella species and their role in extracellular electron transfer. [http://aem.asm.org/content/74/3/615.full Appl. Environ. Microbiol. 74:615–623].
Bacher A., et al. (2001). Biosynthesis of riboflavin. [http://www.ncbi.nlm.nih.gov/pubmed/11153262 Vitam. Horm. 61:1–49.]
Tesliar G. E., Shavlovskii G. M. (1983). Localization of the genes coding for GTP cyclohydrolase II and riboflavin synthase on the chromosome of Escherichia coli K-12. Tsitol. Genet. 17:54–56. (In Russian.)
Seong Han Lim, Jong Soo Choi and Enoch Y. (2001). Park Microbial Production of Riboflavin Using Riboflavin Overproducers, Ashbya gossypii, Bacillus subtilis, and Candida famate: An Overview.[http://www.bbe.or.kr/storage/journal/BBE/6_2/6657/articlefile/article.pdf Biotechnol. Bioprocess Eng., 6: 75-88]
 "
"