Team:ETH Zurich/Experiments 2
From 2013.igem.org
| Line 9: | Line 9: | ||
<h1>Diffusion tests using AHL and a spiral receiver cell set-up</h1> | <h1>Diffusion tests using AHL and a spiral receiver cell set-up</h1> | ||
| - | [[File:AHLdiffusion_5h.png|650px|center|thumb| <b> Figure 2.1 Spiral diffusion experiment after 5h of incubation using the J09855 (P<sub>lac</sub> LuxR P<sub>luxR</sub>) construct and a GFP reporter.</b> On the left image (1) shows the picture at 395 nm excitation wavelength, and on the right image (2) the | + | |
| + | It is vital to know the exact diffusion pattern of AHL from the mine to have an idea of the placement of the colonies on the agar-mine field. By studying the diffusion time and distance of AHL around the sender, we were able to confirm the honey-comb pattern as the design of the mine grid. | ||
| + | |||
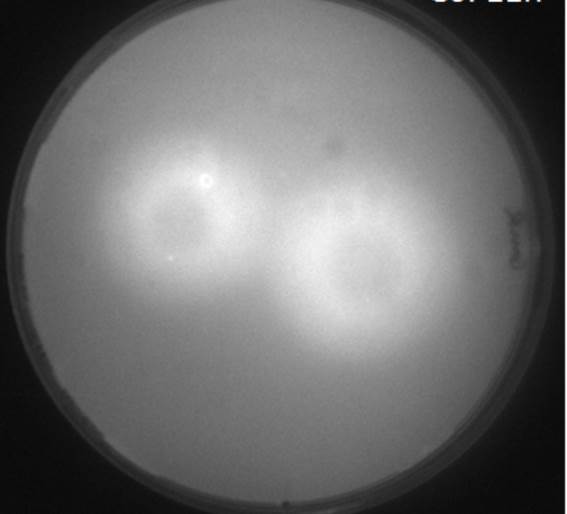
| + | [[File:AHLdiffusion_5h.png|650px|center|thumb| <b> Figure 2.1 Spiral diffusion experiment after 5h of incubation using the J09855 (P<sub>lac</sub> LuxR P<sub>luxR</sub>) construct and a GFP reporter.</b> On the left image (1) shows the picture at 395 nm excitation wavelength, and on the right image (2) the grey-scale of image (1). A drop of AHL (10 uM, 100 uM, 1 mM) was placed on the central colony.]] | ||
[[File:AHLdiffusion_23h.png|650px|center|thumb| <b> Figure 2.2 Spiral diffusion experiment after 23h of incubation using the J09855 construct and a GFP reporter.</b> On the left image (1) shows the picture at 395nm excitation wavelength, and on the right image (2) the greyscale of image (1). A drop of AHL (10 uM, 100uM, 1mM) was placed on the central colony.]] | [[File:AHLdiffusion_23h.png|650px|center|thumb| <b> Figure 2.2 Spiral diffusion experiment after 23h of incubation using the J09855 construct and a GFP reporter.</b> On the left image (1) shows the picture at 395nm excitation wavelength, and on the right image (2) the greyscale of image (1). A drop of AHL (10 uM, 100uM, 1mM) was placed on the central colony.]] | ||
| - | <p align= "justify">The figures 2.1 and 2.2 show diffusion tests using receiver cells with GFP as reporter expressed with part([http://parts.igem.org/Part:BBa_J09855 J09855]). As our sender cells secrete AHL, initial diffusion tests were performed to determine the AHL interaction in the non-mines. We studied the GFP expression in the non-mines in a spiral with increasing distance from the source of diffusion with 1uM, 10uM, 10mM. The origin of diffusion: a drop of AHL was pipetted on the central colony. Figure 2.1 after 5 h of incubation and Figure 2.2 after 23 h of incubation at 37°C. The progression of the AHL throught the agar was interpreted via the GFP fluorescence | + | <p align= "justify">The figures 2.1 and 2.2 show diffusion tests using receiver cells with GFP as reporter expressed with part([http://parts.igem.org/Part:BBa_J09855 J09855]). As our sender cells secrete AHL, initial diffusion tests were performed to determine the AHL interaction in the non-mines. We studied the GFP expression in the non-mines in a spiral with increasing distance from the source of diffusion with 1uM, 10uM, 10mM. The origin of diffusion: a drop of AHL was pipetted on the central colony. Figure 2.1 after 5 h of incubation and Figure 2.2 after 23 h of incubation at 37°C. The progression of the AHL throught the agar was interpreted via the GFP fluorescence.(If you want to know more about the methods please click [https://2013.igem.org/Team:ETH_Zurich/Materials#spiraldiffusion here].) From these experiments, a preliminary idea about the diffusion time and distance of AHL was achieved. In order to determine of diffusion pattern in the game set-up, we tested the sender cells which produced AHL constitutively.(see below Diffusion tests using sender-receiver set-up).</p> |
| - | + | ||
<br clear="all"> | <br clear="all"> | ||
Revision as of 18:45, 28 October 2013
Contents |
Signaling molecule AHL
N-3-Oxo-Hexanoyl-l-Homoserine Lactone belongs to the family of Acylated Homoserine Lactones (AHL).
In our project , we use the LuxI-LuxR quorum sensing system to drive the signal from the sender to the receiver cells. The LuxI sender construct produces AHL. The AHL diffuses in the agar to reach the receiver cells containing LuxR which in turn triggers the hydrolase expression in the receiver colonies. The receiver cells comprise promoters that are tuned to express specific hydrolases depending on the amount of incoming AHL. The AHL diffusion is very important in that it drives the enzyme (hydrolase) expression in the non-mines depending on different high pass filters. The AHL concentration processed by the receiver cells depends on the number of mine colonies. Through an enzyme-susbtrate reaction that generates a colored product the player obtains information about the number of mines surrounding a non-mine.
Diffusion tests using AHL and a spiral receiver cell set-up
It is vital to know the exact diffusion pattern of AHL from the mine to have an idea of the placement of the colonies on the agar-mine field. By studying the diffusion time and distance of AHL around the sender, we were able to confirm the honey-comb pattern as the design of the mine grid.
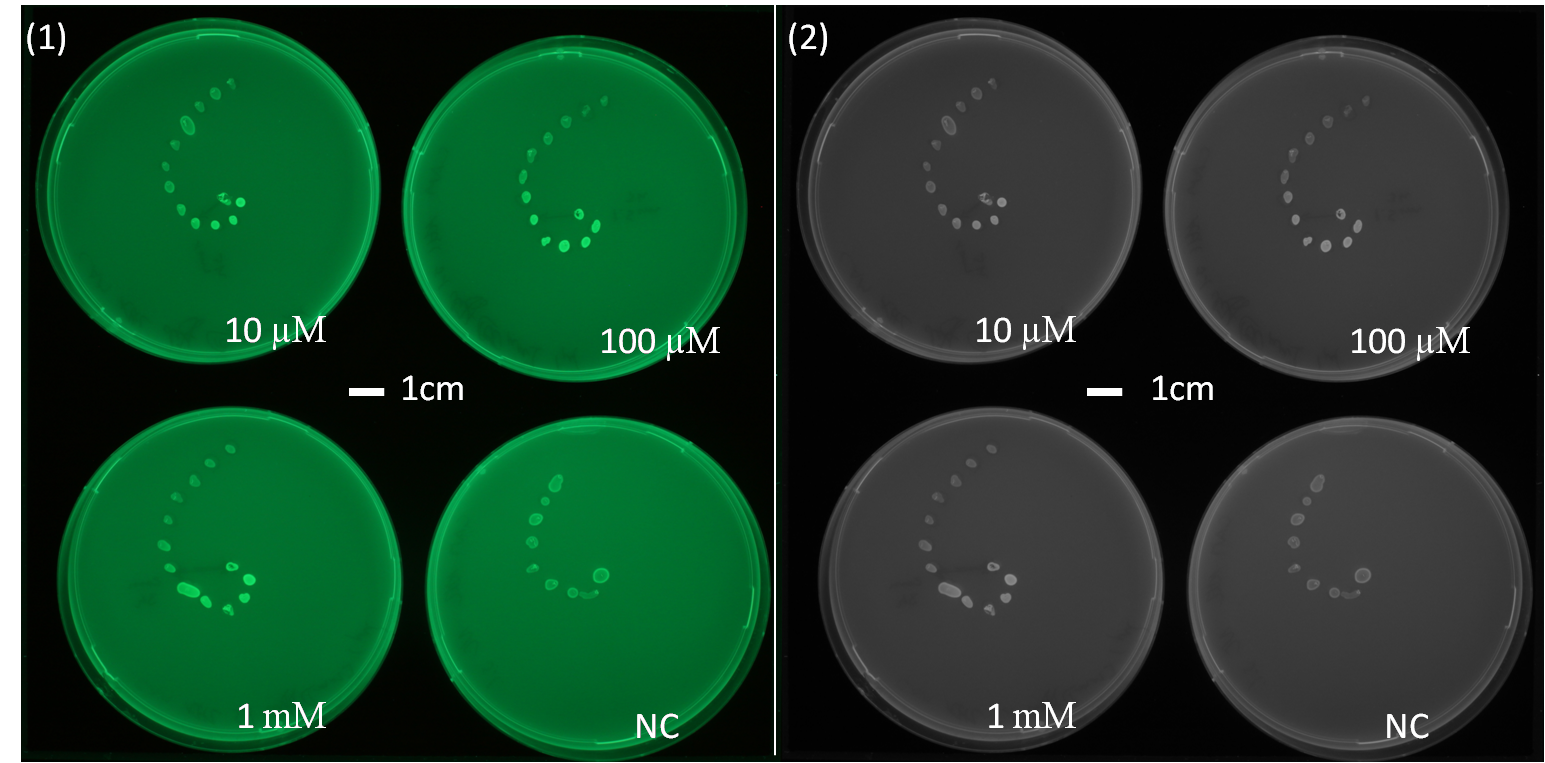
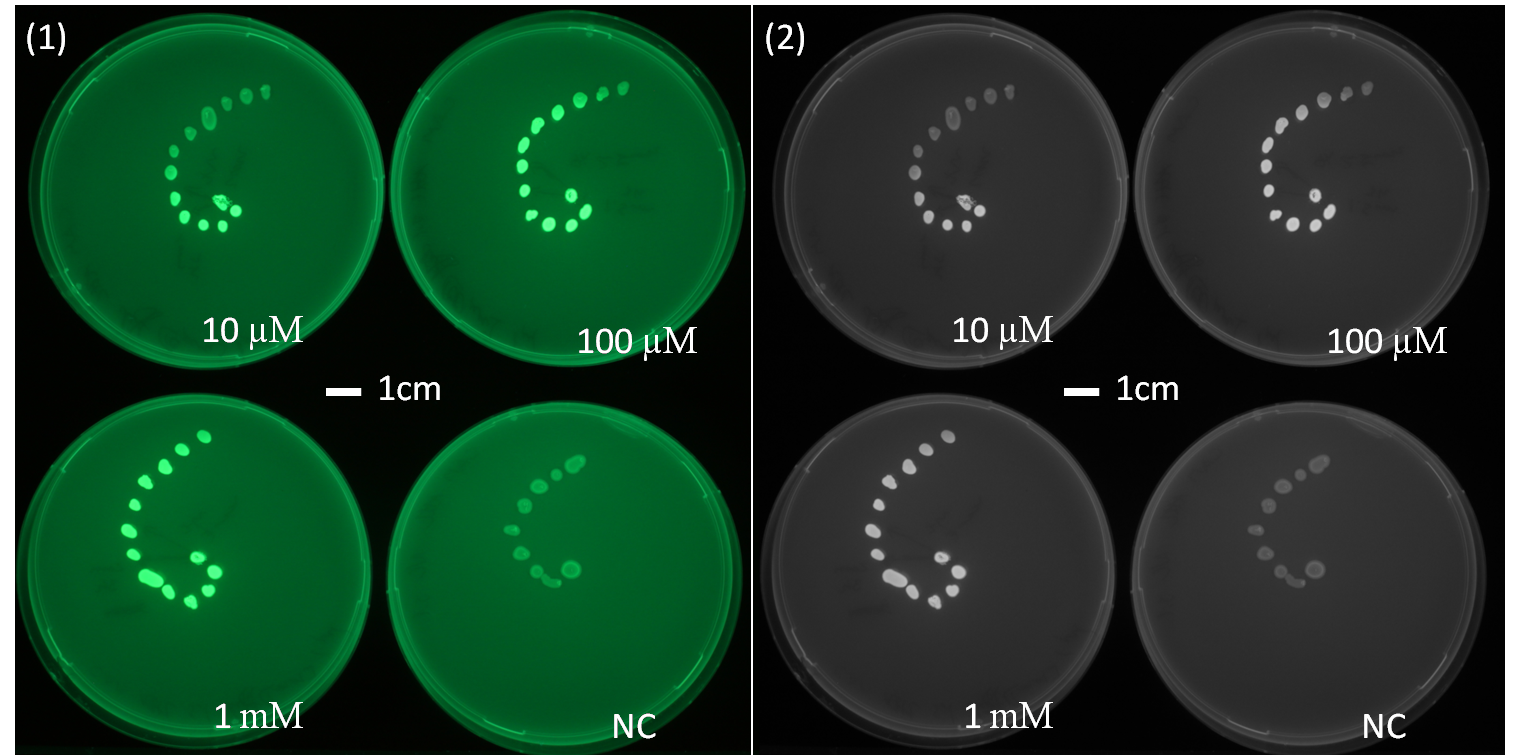
The figures 2.1 and 2.2 show diffusion tests using receiver cells with GFP as reporter expressed with part([http://parts.igem.org/Part:BBa_J09855 J09855]). As our sender cells secrete AHL, initial diffusion tests were performed to determine the AHL interaction in the non-mines. We studied the GFP expression in the non-mines in a spiral with increasing distance from the source of diffusion with 1uM, 10uM, 10mM. The origin of diffusion: a drop of AHL was pipetted on the central colony. Figure 2.1 after 5 h of incubation and Figure 2.2 after 23 h of incubation at 37°C. The progression of the AHL throught the agar was interpreted via the GFP fluorescence.(If you want to know more about the methods please click here.) From these experiments, a preliminary idea about the diffusion time and distance of AHL was achieved. In order to determine of diffusion pattern in the game set-up, we tested the sender cells which produced AHL constitutively.(see below Diffusion tests using sender-receiver set-up).
Diffusion tests using sender-receiver set-up
In our biological circuit design, the sender secretes signalling molecule AHL. This molecule diffuses in and out of cells and thereby communicates between cells. As the AHL molecule diffuses it enters the neighboring non-mine cells. The non-mine cells are equipped with promoters that have different AHL sensitivities. This communication between mines and non-mines is vital in order to express the different output hydrolases. This is because the hydrolases are expressed under the control of PLuxR promoters which are induced by AHL that are diffused from the mines. Hence diffusion tests were performed to test diffusion time and distance of AHL from the sender to the receiver.
Diffusion tests of AHL in double layer agar
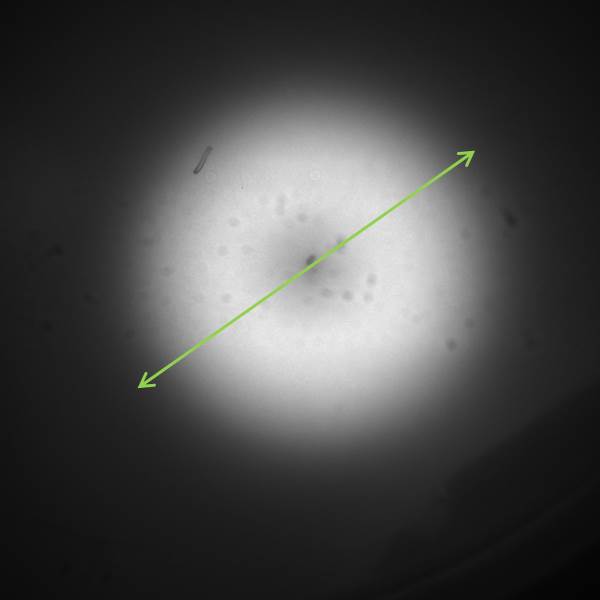
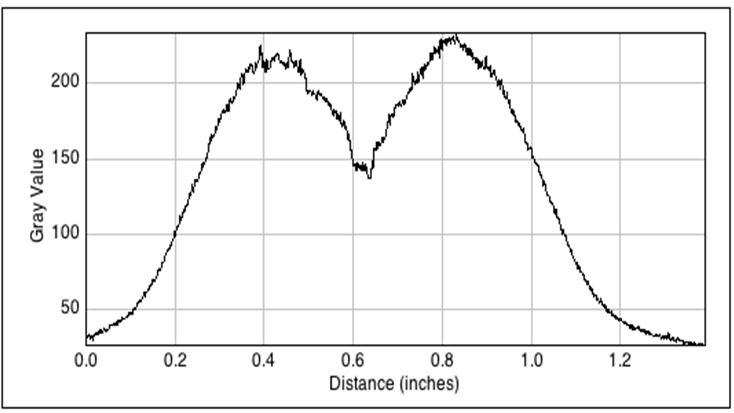
In order to visualize the AHL diffusion, experiments were carried out, using GFP as reporter. We started with using the part [http://parts.igem.org/Part:BBa_J09855 BBa_J09855] cloned with GFP as our receiver. We tested the sender with part [http://parts.igem.org/Part:BBa_K805016 BBa_K805016] under a constitutive promoter. double-layer agar tests (See protocol) were performed with the GFP receiver cells spread evenly as a top layer agar, and the 1.5 μl sender cells as source of signal AHL. The diffusion pattern was measured over 12 hours with a molecular imaging system. The distance of diffusion was noted as 1.5 cm as the radial diffusion distance across the sender colony. The time and distance data from these experiments were used for the spatio-temporal model of the AHL diffusion.
The image to the left shows the GFP fluorescence in a double layer agar experiment after 11 hours. In order to quantify the time and distance of diffusion of AHL from the sender to the GFP receiver, we analyzed the scanned image of the plate with the image processing program ImageJ . The analysis from ImageJ is shown in the picture to the right. The picture shows the GFP fluorescence in gray scale with distance in inches. The distance of diffusion can be seen as 1,2 inches, that is nearly 3 cm. Hence, for the further experiments, the colonies were placed apart from each other at a distance of 1.5 cm in a hexagonal manner.
 "
"