Team:ETH Zurich/Experiments 6
From 2013.igem.org
| Line 15: | Line 15: | ||
<p align= "justify"> The wild-type plux promoter was mutated through [https://2013.igem.org/Team:ETH_Zurich/Experiments_5 site-saturation mutagenesis] | <p align= "justify"> The wild-type plux promoter was mutated through [https://2013.igem.org/Team:ETH_Zurich/Experiments_5 site-saturation mutagenesis] | ||
| - | to achieve different high-pass filters with varying OHHL sensitivities. The OHHL sensitivity of the mutated promoter was compared with the wild-type promoter with grid tests as described above. From the grid tests, it observed that the OHHL sensitivity was too low with respect to wild-type. No induction of the GFP receiver was observed even after 21 hours of incubation. These results | + | to achieve different high-pass filters with varying OHHL sensitivities. The OHHL sensitivity of the mutated promoter was compared with the wild-type promoter with grid tests as described above. From the grid tests, it observed that the OHHL sensitivity was too low with respect to wild-type. No induction of the GFP receiver was observed even after 21 hours of incubation. The model predicted that expression was seen after 15hours. These results can be viewed [[https://2013.igem.org/Team:ETH_Zurich/Modelingmodel here]]. <br> |
The OHHL sensitivity was too low and hence additional tests were carried out for more promoter mutants by back mutating to the wild-type, in order to recover some sensitivity.</p> | The OHHL sensitivity was too low and hence additional tests were carried out for more promoter mutants by back mutating to the wild-type, in order to recover some sensitivity.</p> | ||
Revision as of 22:10, 4 October 2013
Contents |
GFP grid tests with sender cells and wild-type pLux receiver constructs
Diffusion experiments were performed to study OHHL diffusion from the sender colonies to the receiver colonies. The colonies were placed in a hexagonal grid pattern such that every colony can have zero, one, two or three mine colonies adjacent to it. Depending on the number of mines around a non-mine colony, more OHHL will be processed in the receiver colonies due to the higher number of mines. 1.5μl of the receiver and sender cultures were plated according to a hexagonal grid pattern on an agar plate. We then investigated the diffusion patterns by scanning the plate with a molecular imaging software. Images of the GFP fluorescence were taken at time intervals of 1.5hours after first detection of fluorescence. Images taken after 11 hours showed GFP saturation.
The picture to the right shows the scanned image of GFP fluorescence after 11 hours of incubation. The green circles mark the mine colonies. The receiver colonies are those that process the OHHL that diffused from the sender colonies. The difference in fluorescence in the receiver colonies correlates directly to the number of adjacent mine colonies. The data from this experiment shows almost complete agreement with the spatio-temporal model.
GFP grid tests with sender cells and mutated pluxR promoter receiver constructs
The wild-type plux promoter was mutated through site-saturation mutagenesis
to achieve different high-pass filters with varying OHHL sensitivities. The OHHL sensitivity of the mutated promoter was compared with the wild-type promoter with grid tests as described above. From the grid tests, it observed that the OHHL sensitivity was too low with respect to wild-type. No induction of the GFP receiver was observed even after 21 hours of incubation. The model predicted that expression was seen after 15hours. These results can be viewed [here].
The OHHL sensitivity was too low and hence additional tests were carried out for more promoter mutants by back mutating to the wild-type, in order to recover some sensitivity.
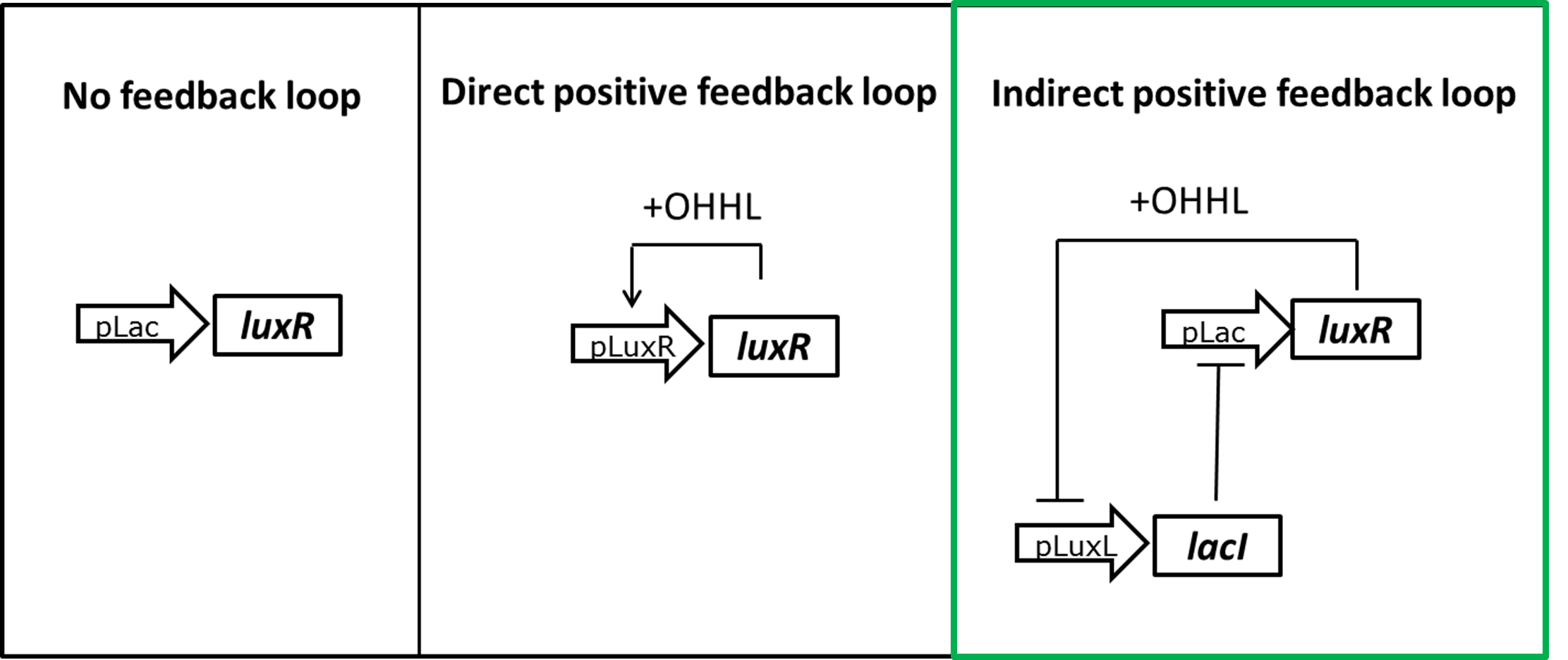
Optimization of LuxR production regulation to reduce basal reporter activation
Already with the GFP reporter we encountered leakiness in the absence of OHHL. We tested the GFP expression of different constructs in liquid culture over time and could show that most of the leakiness comes from activation of the pLux promoter of LuxR alone, meaning in the absence of OHHL (Figure 3). Expecting the hydrolase reporter system to be even more sensitive we designed different strategies to reduce the level of LuxR in the uninduced state.
 "
"