Team:ETH Zurich/Experiments 3
From 2013.igem.org
| Line 41: | Line 41: | ||
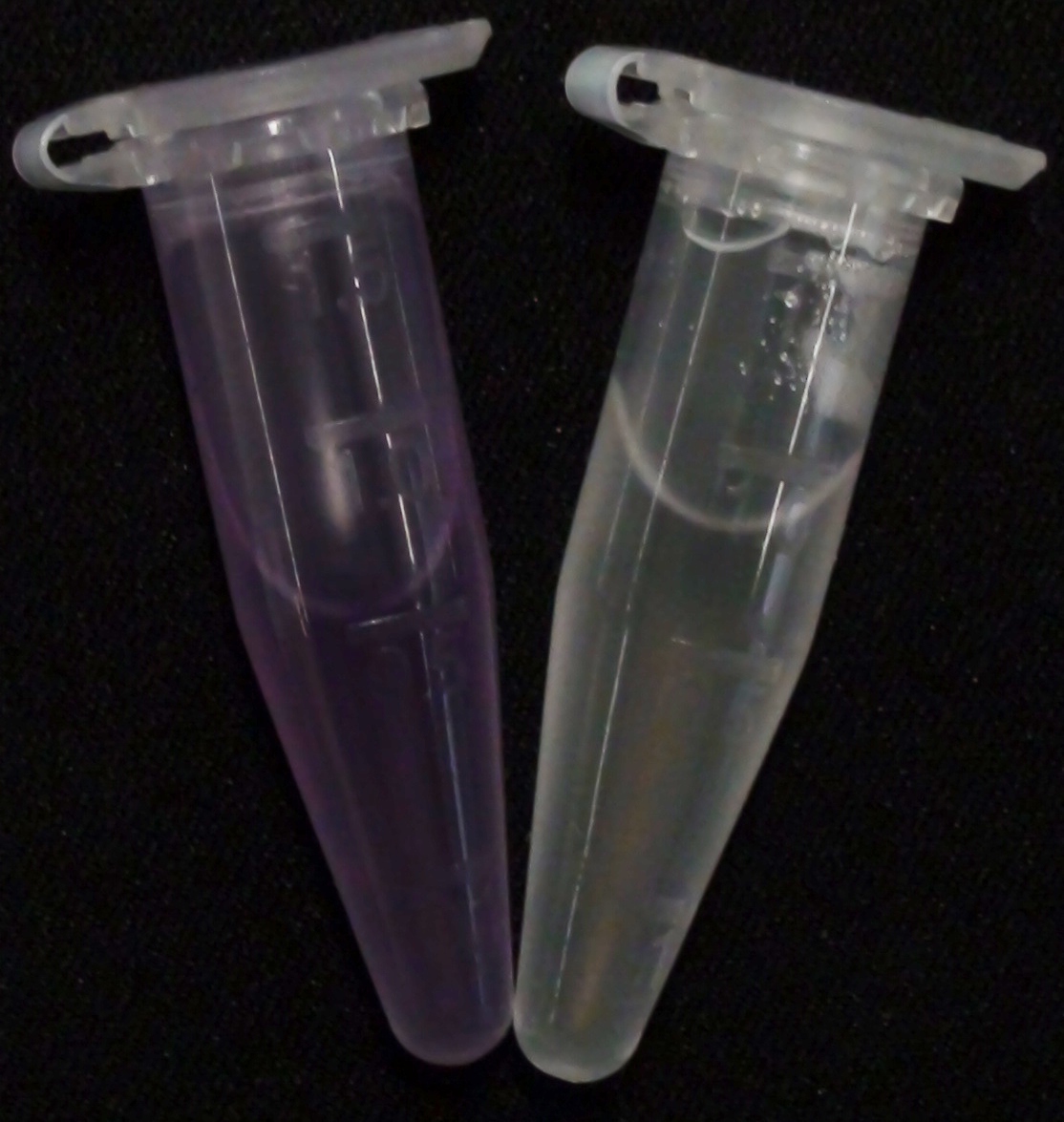
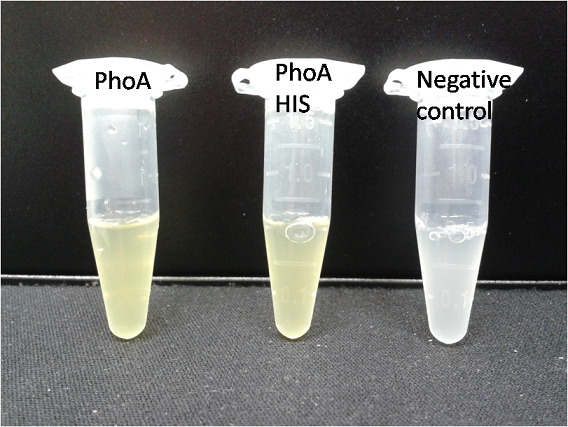
[[File:PhoA_PhoA_his_compared_colored.png|thumb|right|200px|<b>Figure 8.</b> Liquid culture from <i>Escherichia coli</i> overexpressing PhoA or PhoA-His respectively after reacting with pNPP. The negative control tube contains liquid culture without pNPP.]] | [[File:PhoA_PhoA_his_compared_colored.png|thumb|right|200px|<b>Figure 8.</b> Liquid culture from <i>Escherichia coli</i> overexpressing PhoA or PhoA-His respectively after reacting with pNPP. The negative control tube contains liquid culture without pNPP.]] | ||
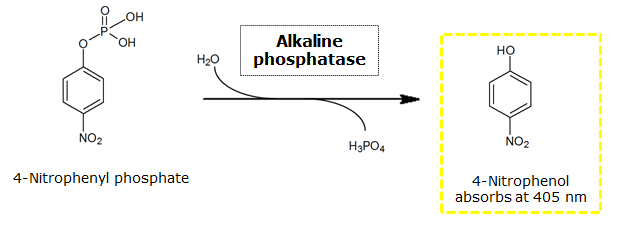
| - | <p align ="justify"><b>Chromogenic assay</b><br>To test the functionality of this PhoA, a liquid culture of <i>Escherichia coli</i> overexpressing this hydrolase was grown until an OD<sub>600</sub> of 0.4 - 0.6 was reached before addition of ''p''-nitrophenyl phosphate (pNPP) to a final concentration of 5 mM. This substrate gives rise to yellow color after hydrolysis by PhoA (Figure 8). In the Colisweeper game, substrates are pipetted onto colonies | + | <p align ="justify"><b>Chromogenic assay</b><br>To test the functionality of this PhoA, a liquid culture of <i>Escherichia coli</i> overexpressing this hydrolase was grown until an OD<sub>600</sub> of 0.4 - 0.6 was reached before addition of ''p''-nitrophenyl phosphate (pNPP dissolved in DMSO) to a final concentration of 5 mM. This substrate gives rise to yellow color after hydrolysis by PhoA (Figure 8). In the Colisweeper game, substrates are pipetted onto colonies, Figure 10 shows color development on colonies five minutes after addition of 1.5 µl of a 0.5 M pNPP solution. |
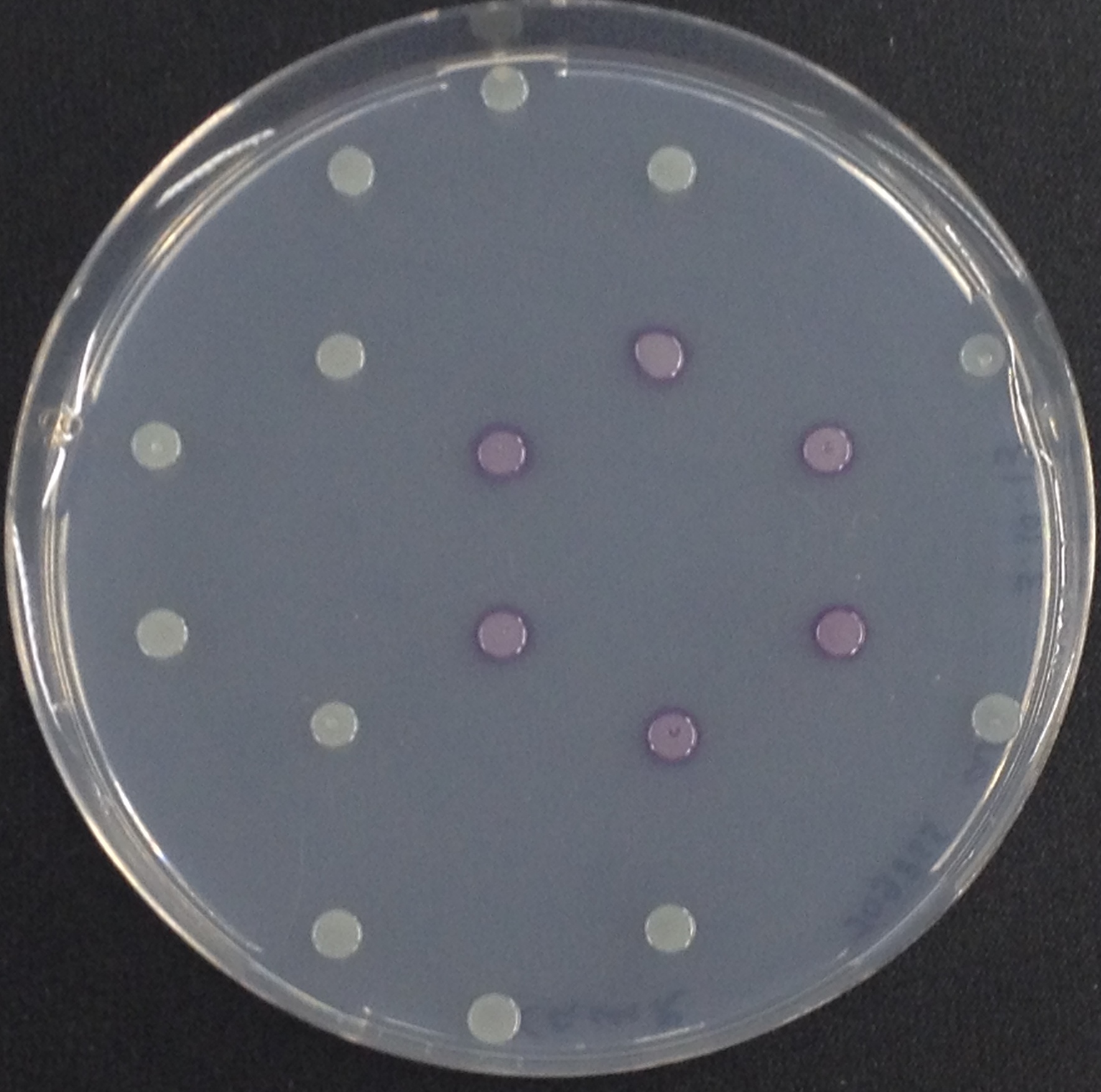
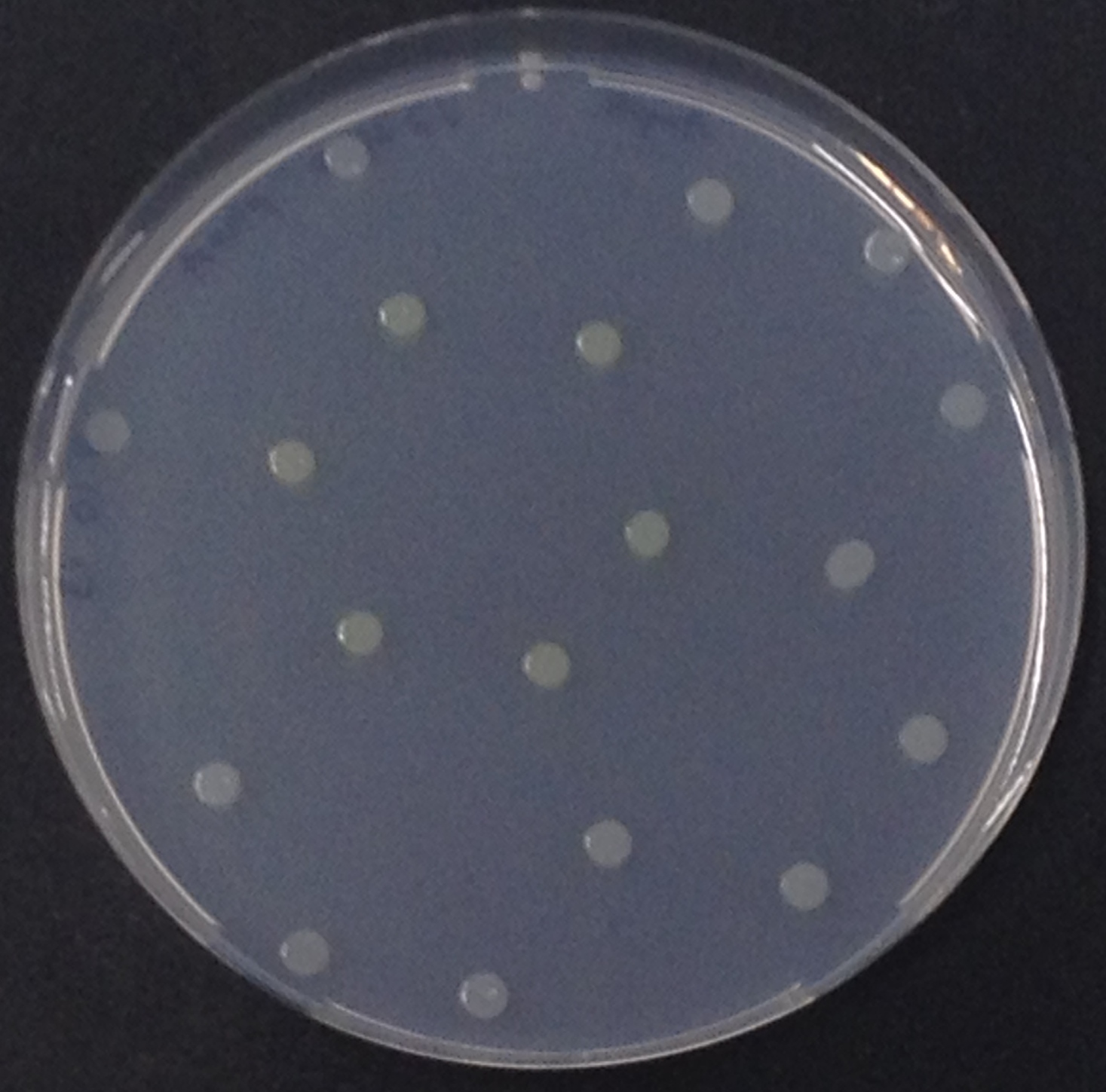
[[File:PhoA and pNPP.jpeg|thumb|right|200px| <b>Figure 10.</b> Colonies of ''Escherichia coli'' overexpressing the ''Citrobacter'' PhoA. Colonies produce yellow color after addition of pNPP.]]</p> | [[File:PhoA and pNPP.jpeg|thumb|right|200px| <b>Figure 10.</b> Colonies of ''Escherichia coli'' overexpressing the ''Citrobacter'' PhoA. Colonies produce yellow color after addition of pNPP.]]</p> | ||
<br> | <br> | ||
| Line 49: | Line 49: | ||
<b>Characterization of PhoA-HIS tagged protein</b> | <b>Characterization of PhoA-HIS tagged protein</b> | ||
[[File:Phoahis.png|left|400px|thumb|<b>Figure 11. PhoA-HIS-tag characterization by western blotting.</b> The red stripe indicates the anti-anti-HIS antibody carrying the red fluorescent dye. The white circles indicate the PhoA-HIS protein in the SDS-PAGE gel as well as on the western blot membrane. The molecular weight of this protein is 53kDa.]] | [[File:Phoahis.png|left|400px|thumb|<b>Figure 11. PhoA-HIS-tag characterization by western blotting.</b> The red stripe indicates the anti-anti-HIS antibody carrying the red fluorescent dye. The white circles indicate the PhoA-HIS protein in the SDS-PAGE gel as well as on the western blot membrane. The molecular weight of this protein is 53kDa.]] | ||
| - | <p align="justify">The PhoA and PhoA with HIS-tag were overexpressed in the ''Escherichia coli'' BL21 DE3 strain, induced by 5 μM IPTG (iso-propy-β-D-1-thiogalactopyranoside) and finally harvested in order to obtain the cell lysate by lysozyme lysis. This cell lysate of both cultures were analyzed next to each other in two SDS-PAGE gels, one for comassie staining (blue gel in the picture) and one for western blotting (black picture) with Anti-6X His tag® antibody from mouse and a second reporter goat anti mouse antibody with a IRDye 680RD. In the picture we can distinguish the PhoA His (53 kDa) on the western blot as well as on the SDS-PAGE gel (see white circles).</p> | + | <p align="justify">We introduced a HIS tag to the PhoA enzyme. The HIS tag enables to purify the enzyme and make a full Michalis Menten studies of PhoA which can give exact values for the model. Adding of a HIS tag can lead to an unfunctional enzyme, so we first tested the functionality of both PhoA and PhoA HIS tag (see Figure 9) with our coloremetric substrat. In a second step we confirmed the presents of the HIS tag by Western Blot: The PhoA and PhoA with HIS-tag were overexpressed in the ''Escherichia coli'' BL21 DE3 strain, induced by 5 μM IPTG (iso-propy-β-D-1-thiogalactopyranoside) and finally harvested in order to obtain the cell lysate by lysozyme lysis. This cell lysate of both cultures were analyzed next to each other in two SDS-PAGE gels, one for comassie staining (blue gel in the picture) and one for western blotting (black picture) with Anti-6X His tag® antibody from mouse and a second reporter goat anti mouse antibody with a IRDye 680RD. In the picture we can distinguish the PhoA His (53 kDa) on the western blot as well as on the SDS-PAGE gel (see white circles).</p> |
<br clear="all"/> | <br clear="all"/> | ||
Revision as of 20:49, 26 October 2013
Contents |
Enzyme-substrate reactions
To generate visible output by adding substrates to colonies in Colisweeper, we made use of orthogonal enzyme-substrate reactions. A set of chromogenic substrates was chosen to produce different colors depending on the abundant hydrolases and thereby to uncover the identity of each colony to the player.
The set of enzyme-substrate pairs chosen for the Colisweeper project, and characterizations of their reactions, are described below. Information on other possible substrates that can be used for the enzymes of the Colisweeper reporter system can be found in the reporter system section.
To characterize the hydrolases used in Colisweeper, we conducted substrate tests in liquid cultures as well as on colonies, enzyme kinetics and detection of HIS-tagged protein by Western Blot. Additionally, crosstalk assays and color overlay tests (multiple enzyme-substrate reactions together) have been performed for this project. An overview of chromogenic substrates used can be found in the Materials section.
Expand the boxes to see the characterization of the hydrolases
Acetyl esterase (Aes)
Chromogenic assay
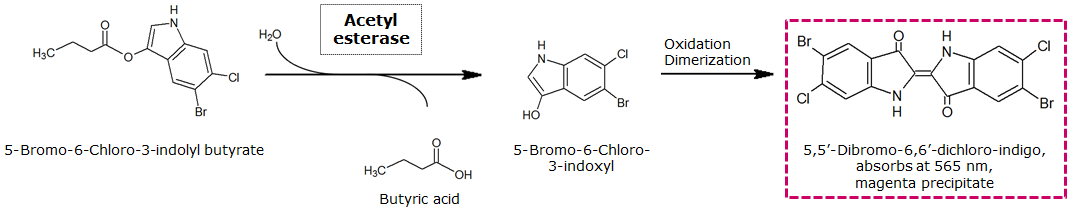
To assess color development after reaction of the enzyme with the chromogenic substrate, a liquid culture of our triple knockout Escherichia coli strain overexpressing Aes was grown until an OD600 of 0.4 - 0.6 was reached before addition of 5-Bromo-6-Chloro-3-indolyl butyrate (magenta butyrate dissolved in DMSO) to a final concentration of 100 µM (see Figure 2). To study the color development in the actual Colisweeper game setup, colonies were plated by pipetting 1.5 µl of the triple knockout Escherichia coli liquid culture (OD600 of 0.4 - 0.6) on an M9 agar plate and incubated for 5 h. Addition of 1.5 µl of 20 mM magenta butyrate onto each colony results in color generation visible after a few minutes at room temperature (see Figure 3).
Enzyme kinetics
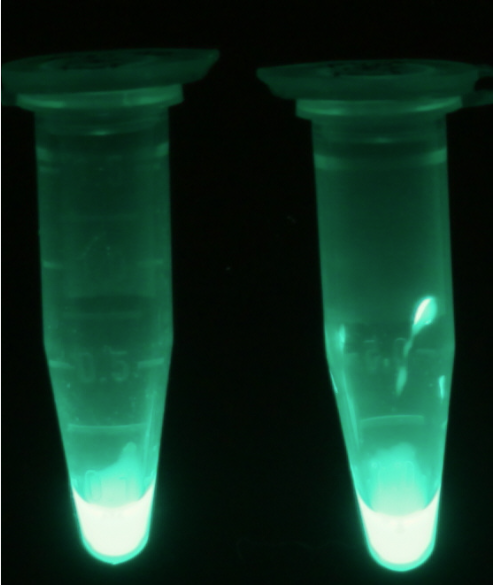
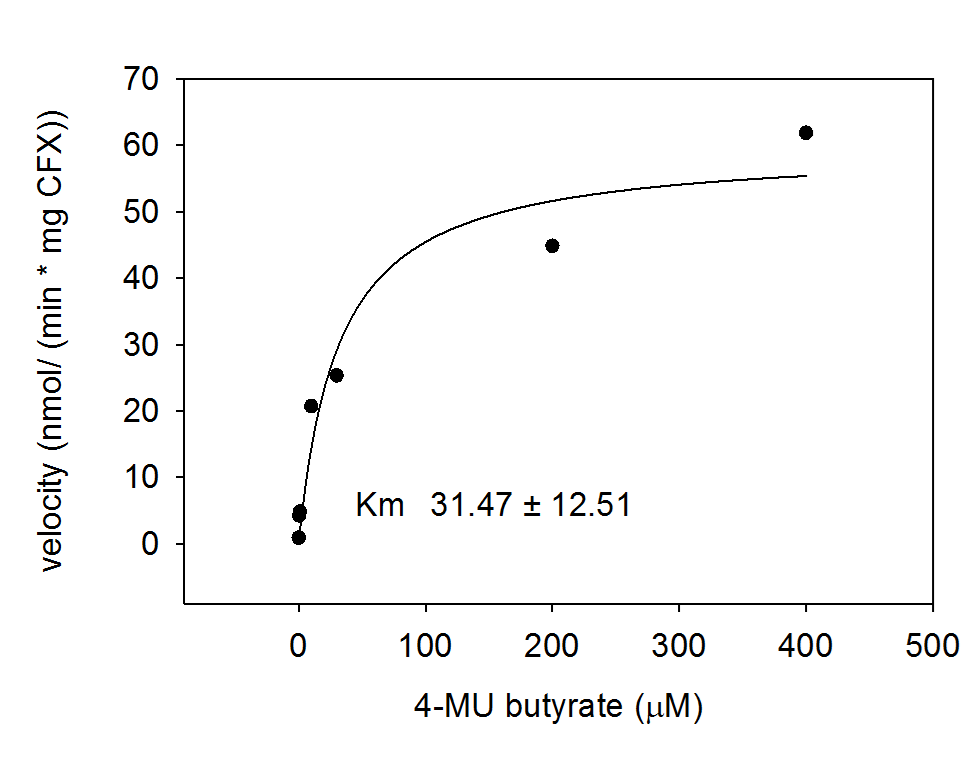
For the kinetics assay, the Escherichia coli lysate overexpressing Aes was tested with the substrate 4-MU-butyrate which after cleavage gives a fluorescent product. The picture on the right (Figure 5) was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
To characterize the enzyme, we conducted Michaelis Menten kinetics to obtain the Km value. Escherichia coli cells were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. The plate reader took measurements at λEx 365 nm and λEm 445 nm and determined a Km value of 31.5 ± 12.5 μM. The obtained data was then evaluated and fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
Alkaline phosphatase (PhoA)
Chromogenic assay
To test the functionality of this PhoA, a liquid culture of Escherichia coli overexpressing this hydrolase was grown until an OD600 of 0.4 - 0.6 was reached before addition of p-nitrophenyl phosphate (pNPP dissolved in DMSO) to a final concentration of 5 mM. This substrate gives rise to yellow color after hydrolysis by PhoA (Figure 8). In the Colisweeper game, substrates are pipetted onto colonies, Figure 10 shows color development on colonies five minutes after addition of 1.5 µl of a 0.5 M pNPP solution.
Characterization of PhoA-HIS tagged protein
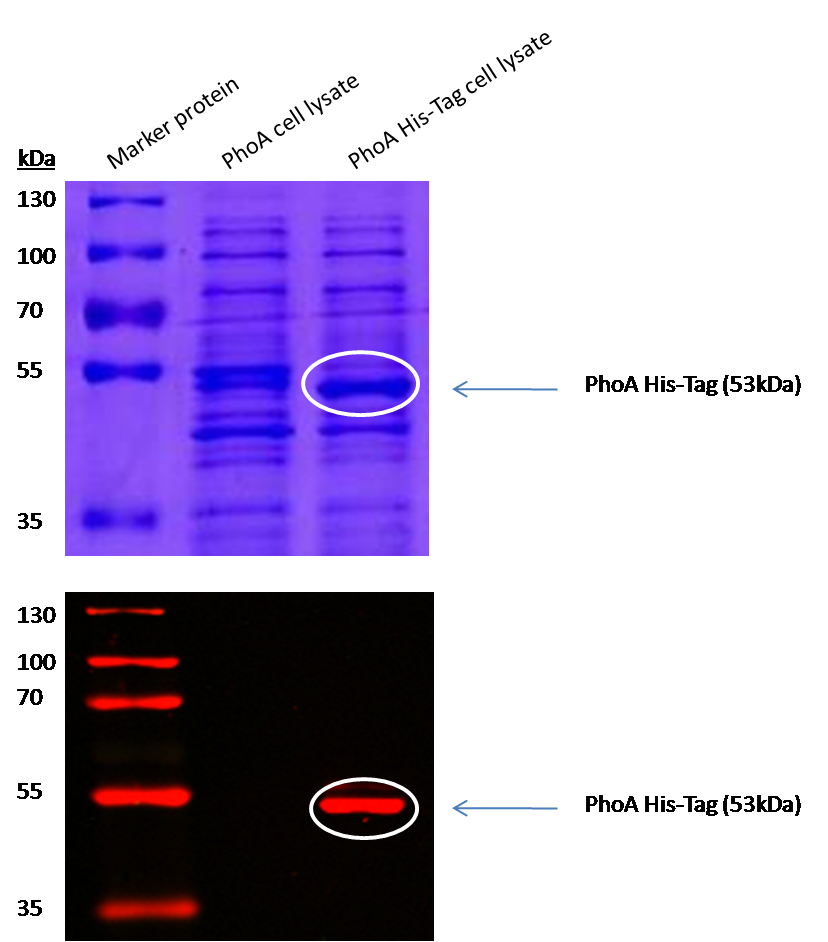
We introduced a HIS tag to the PhoA enzyme. The HIS tag enables to purify the enzyme and make a full Michalis Menten studies of PhoA which can give exact values for the model. Adding of a HIS tag can lead to an unfunctional enzyme, so we first tested the functionality of both PhoA and PhoA HIS tag (see Figure 9) with our coloremetric substrat. In a second step we confirmed the presents of the HIS tag by Western Blot: The PhoA and PhoA with HIS-tag were overexpressed in the Escherichia coli BL21 DE3 strain, induced by 5 μM IPTG (iso-propy-β-D-1-thiogalactopyranoside) and finally harvested in order to obtain the cell lysate by lysozyme lysis. This cell lysate of both cultures were analyzed next to each other in two SDS-PAGE gels, one for comassie staining (blue gel in the picture) and one for western blotting (black picture) with Anti-6X His tag® antibody from mouse and a second reporter goat anti mouse antibody with a IRDye 680RD. In the picture we can distinguish the PhoA His (53 kDa) on the western blot as well as on the SDS-PAGE gel (see white circles).
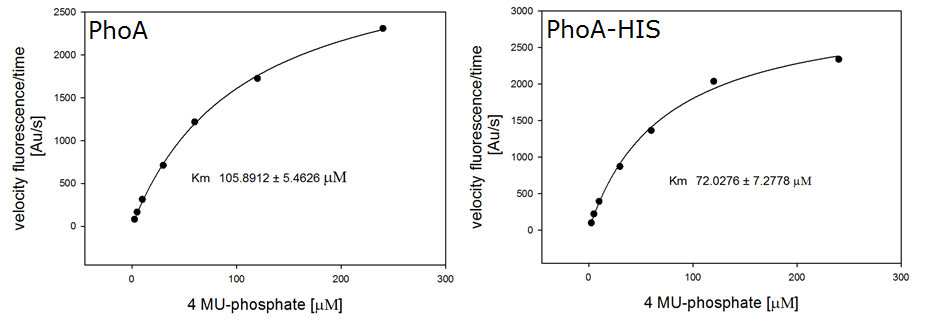
Enzyme kinetics
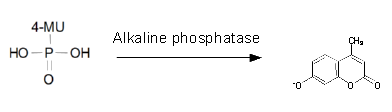
For the kinetics assay, we tested both the Citrobacter phoA and phoA-HIS encoded proteins with the fluorescent substrate 4-MU-phosphate. The image in Figure 12 was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
To characterize the enzymes, we conducted fluorometric assays to obtain Km values. Escherichia coli were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. The plate reader took measurements at λEx 365 nm and λEm 445 nm, determining a Km values of 105.9 ± 5.3 μM for PhoA and 72.0 ± 7.3 μM for Pho-HIS.
The obtained data was evaluated and finally fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
β-Galactosidase (LacZ)
Chromogenic assay
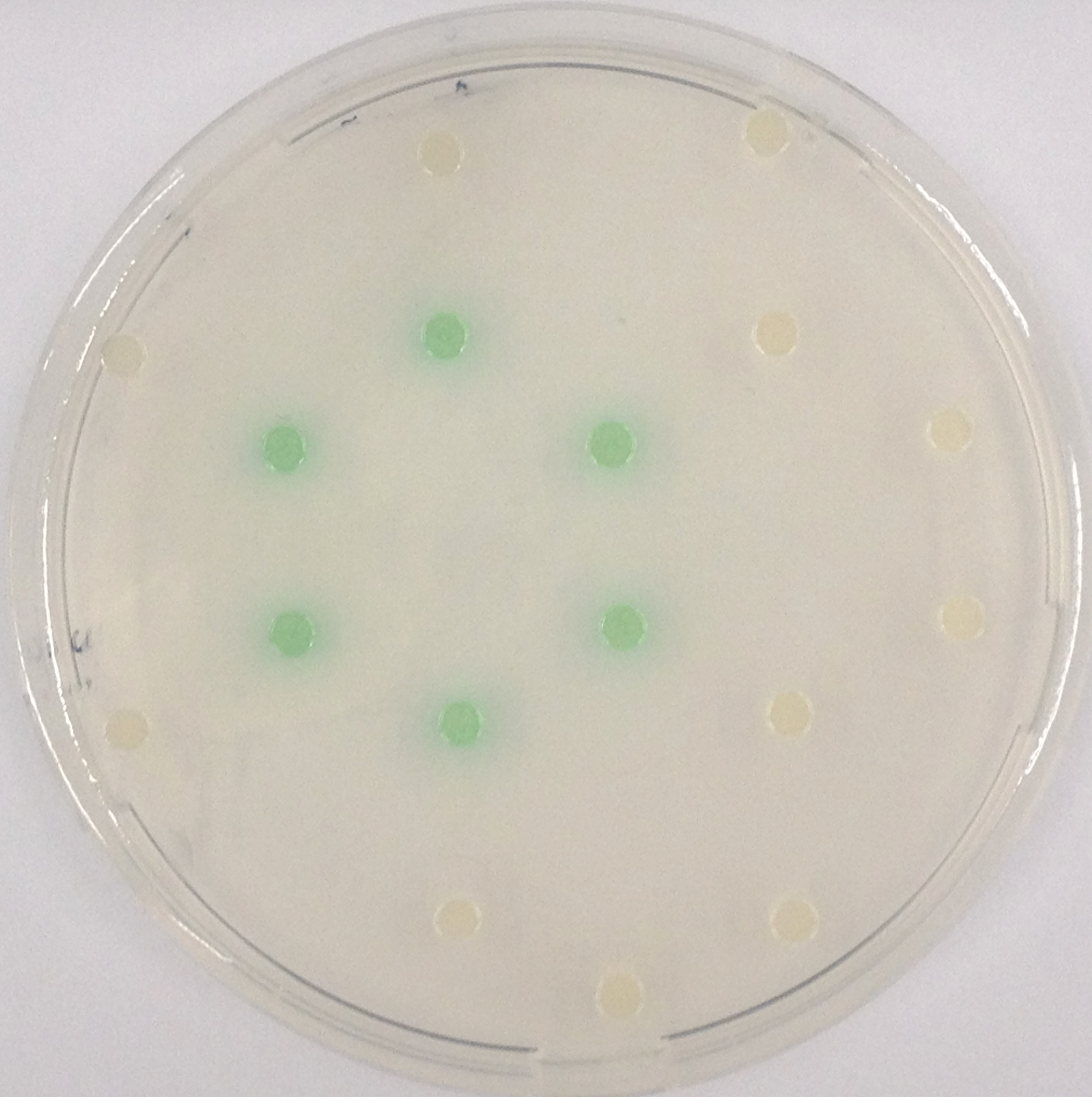
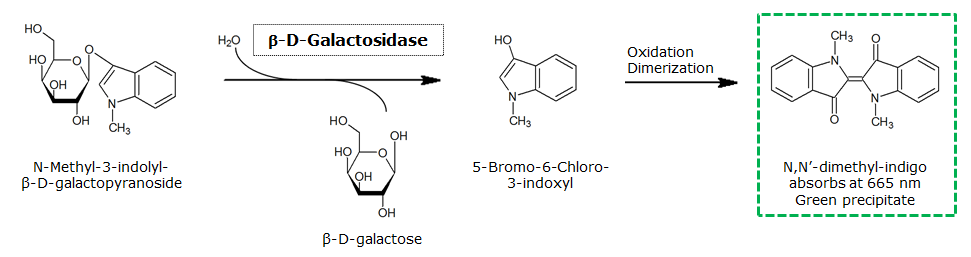
The β-Galactosidase is natively expressed in the triple knockout Escherichia coli strain used in Colisweeper. Its commonly used chromogenic substrate is 5-Bromo-4-Chloro-3-indolyl-β-D-Galactopyranoside (X-Gal), which yields a blue-colored precipitate (Figure 15, middle). In Colisweeper, this protein catalyzes hydrolysis of the flagging substrate, which is N-Methyl-3-indolyl-β-D-Galactopyranoside (Green-β-D-Gal) and produces a green color upon cleavage of the chromophore (Figure 15, left tube). For the color assay in liquid culuture, we used Escherichia coli cultures grown to an OD600 of 0.4 - 0.6, then added the substrates to a final concentration of 50 mM.
β-Glucuronidase (GusA)
Chromogenic assay
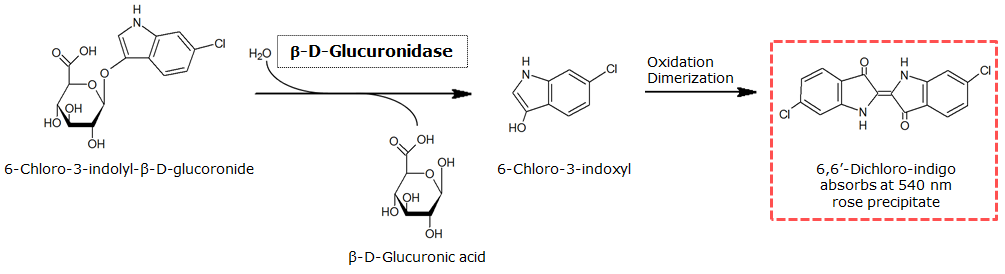
To characterize the reaction of GusA with its substrate, a liquid culture of Escherichia coli overexpressing GusA was grown until an OD600 of 0.4 - 0.6 was reached before addition of 6-Chloro-3-indolyl-β-glucuronide (Salmon-Gluc), a substrate for GusA which produces a rose/salmon color after cleavage (Figure 18). The final concentration of the substrate in liquid culture was 1 mM and the conversion of this substrate was done by incubation at 37°C.
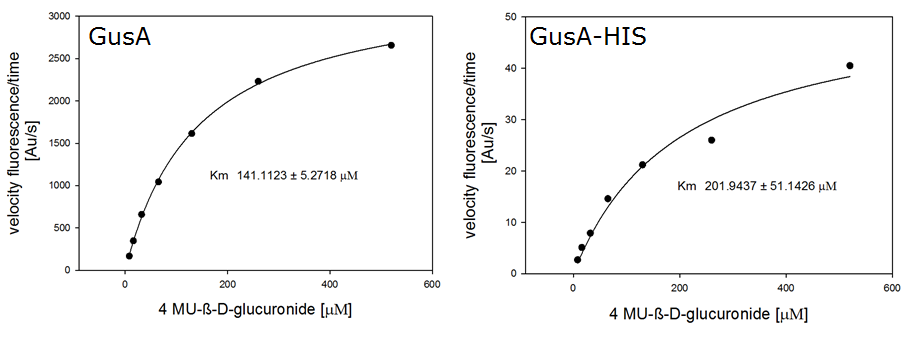
Enzyme kinetics
Chromogenic assay
For the kinetics assay, we tested both gusA and gusA-HIS encoded proteins with the fluorescent substrate 4-MU-β-D-Glucuronide. The picture on the right (Figure 20) was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
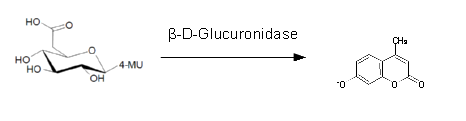
For the enzyme kinetics of GusA, we conducted fluorometric assays to obtain the Km value. Escherichia coli overexpressing GusA were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. A plate reader took measurements at λEx 365 nm and λEm 445 nm, and determined a Km value of 141.1 ± 5.3 μM for GusA, and 201.9 ± 51.1 μM for GusA-HIS. The obtained data was evaluated and finally fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
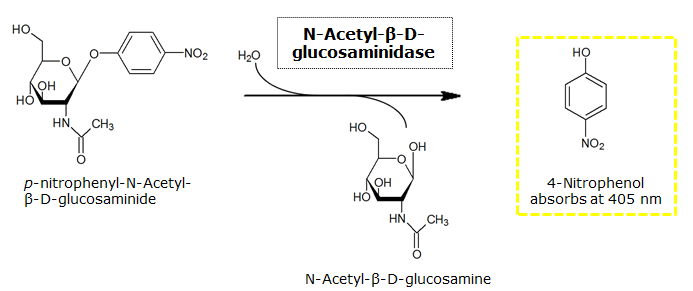
β-N-Acetylglucosaminidase (NagZ)
Chromogenic assay
NagZ was proposed to be constitutively expressed in the mine colonies, processing its blue color-yielding substrate 5-Bromo-4-Chloro-3-indolyl-β-N-acetylglucosaminide (X-GluNAc), as shown in Figure 25. However, we could not initially manage to get color from the reaction with this enzyme-substrate pair. Using p-nitrophenyl-β-N-acetylglucosaminide (pNP-GluNAc) at an end concentration of 0.01 mM in an Escherichia coli liquid culture with an OD600 of 0.4 - 0.6, development of yellow color could be observed (Figure 23), confirming that NagZ is expressed in our mine cells.
According to Orenga et al. (1), the substrate incorporating the indoxyl chromophore is less preferentially hydrolyzed by bacterial NagZ in comparison to yeast NagZ, and more preferentially hydrolyzes the alizarine-based substrate they applied. To increase hydrolysis of the indoxyl-based substrate, we added the substrate to a liquid culture supplemented with BSA for stabilization of the enzyme (2), which enhanced hydrolysis by NagZ dramatically. The end concentration in liquid culture of both X-GluNAc and 5-Bromo-6-Chloro-3-indolyl-β-N-acetylglucosaminide (Magenta-GluNAc), a magenta color-yielding substrate for NagZ that we found by chance :), were at 1 mM (Figure 23).
Crosstalk
To ensure orthogonality between the enzyme-substrate reactions used in Colisweeper, a crosstalk test has been done to ensure that all overexpressed enzymes specifically cleave their assigned substrate.
Color overlay
To play Colisweeper, colors for each colony identity have to be clearly distinguishable by eye. Because we are using high-pass filters to differentially process certain AHL levels, we had to make sure that a mix of cleaved product would show distinguishable colors.
References
(1) Orenga S, Roger-Dalbert C, James A, Perry J, US Patent 20090017481 (2009).
(2) Magnelli PE, Bielik A, Guthrie E, Methods Mol. Biol., 801, 189-211 (2011).
Absorption: Biosynth
 "
"