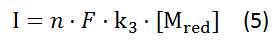Team:Bielefeld-Germany/Modelling
From 2013.igem.org
| Line 58: | Line 58: | ||
<p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Reduction">Mediator<br> Reduction</a></p></div> | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Reduction">Mediator<br> Reduction</a></p></div> | ||
<div class="bigbutton"> | <div class="bigbutton"> | ||
| - | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling | + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Oxidation">Mediator<br> Oxidation</a></p></div> |
<div class="bigbutton"> | <div class="bigbutton"> | ||
<p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Optimal">Optimal<br> conditions</a></p></div> | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Optimal">Optimal<br> conditions</a></p></div> | ||
Revision as of 21:20, 27 October 2013
Modelling
Approach
In a Microbial Fuel Cell (MFC) the chemical energy is transformed into the electrical energy via a cascade of electrochemical reactions. Electrons are produced in the metabolic pathways and can be extracted from the cell and concentrated at the electrode by the electric potential differences. Alternatively the electrons can be transferred to the oxidized mediator molecules that transfer them further to the electrode. There is a variety of parameters and interactions that influence electricity generation. Therefore, there is the need to identify the bottleneck reactions and limiting factors. This approach reduces the complexity of the analysis and can give a deeper insight on the most important processes involved in the electricity generation.
In our theoretical analysis the focus was set to three bottleneck reactions involved in the electron flow from the metabolism of the bacterial cells to the cathode:
- Generation of intermediates NADH/H+ in the metabolic pathway of E.coli
- Reduction of oxidized mediators via the intermediate NADH
- Transfer of the electrons from reduced mediator to the electrode
Mediator Oxidation
In a third electrochemical reaction the reduced mediator is regenerated at the electrode.This electrochemical oxidation at the anode surface occurs as shown in equation:
,where Mred is the reduced mediator,
Mox the oxidized mediator and
k3 is the rate constant of the reaction
Then the current output can be calculated based on formula according to the Faraday's law:
,where I is the current density [A]
[Mred] is the concentration of reduced mediator in the chamber
n is the number of electrons taking part in the electrode reaction,
F is the Faradays constant (96 500 C) and
k3 is the reaction rate, mentioned above.
 "
"