Team:ETH Zurich/Experiments 2
From 2013.igem.org
| Line 28: | Line 28: | ||
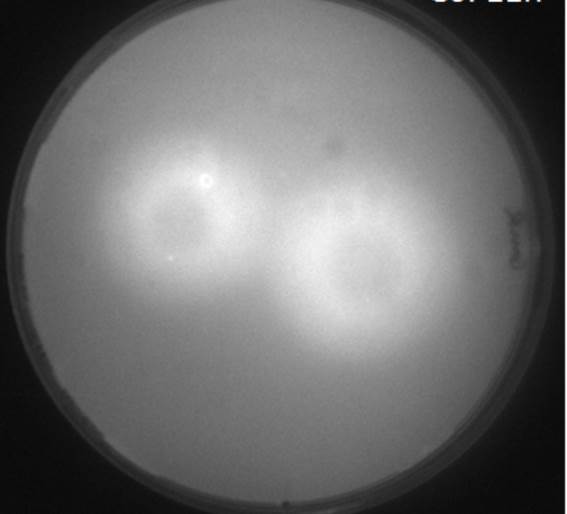
[[File:Diffusion.jpg|left|200px|thumb|<b>Figure 3.2: Scanned image of GFP fluorescence of AHL Diffusion test </b> to check diffusion time and distance ]] | [[File:Diffusion.jpg|left|200px|thumb|<b>Figure 3.2: Scanned image of GFP fluorescence of AHL Diffusion test </b> to check diffusion time and distance ]] | ||
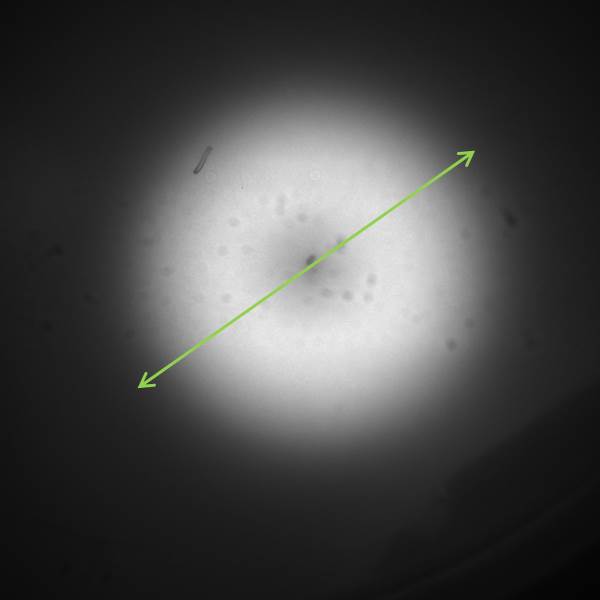
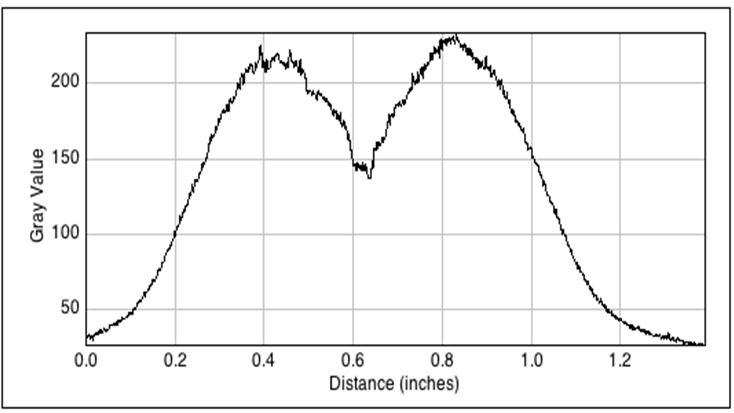
[[File:GFP_profile.png|right|360px|thumb|<b>Figure 3.3: <i>ImageJ</i> analysis of fluorescence peak showing radial diffusion around one sender colony</b>]] | [[File:GFP_profile.png|right|360px|thumb|<b>Figure 3.3: <i>ImageJ</i> analysis of fluorescence peak showing radial diffusion around one sender colony</b>]] | ||
| - | <p align= "justify">The image to the left shows the GFP fluorescence in a double layer agar experiment after 11 hours. In order to quantify the time and distance of diffusion of AHL from the sender to the GFP receiver, we analyzed the scanned image of the plate with the image processing program <i>ImageJ</i> . The analysis from <i>ImageJ</i> is shown in the picture to the right. The picture shows the GFP fluorescence in gray scale with distance in inches. The distance of diffusion can be seen as 1,2 inches, that is nearly 3 cm. Hence, for the further experiments, the colonies were placed apart from each other at a distance of '''1 | + | <p align= "justify">The image to the left shows the GFP fluorescence in a double layer agar experiment after 11 hours. In order to quantify the time and distance of diffusion of AHL from the sender to the GFP receiver, we analyzed the scanned image of the plate with the image processing program <i>ImageJ</i> . The analysis from <i>ImageJ</i> is shown in the picture to the right. The picture shows the GFP fluorescence in gray scale with distance in inches. The distance of diffusion can be seen as 1,2 inches, that is nearly 3 cm. Hence, for the further experiments, the colonies were placed apart from each other at a distance of '''1.5 cm''' in a hexagonal manner.</p> |
<br clear="all"/> | <br clear="all"/> | ||
Revision as of 16:41, 28 October 2013
Contents |
Signaling molecule AHL
N-3-Oxo-Hexanoyl-l-Homoserine Lactone belongs to the family of Acylated Homoserine Lactones (AHL).
In our project , we use the LuxI-LuxR quorum sensing system to drive the signal from the sender to the receiver cells. The LuxI sender construct produces AHL. The AHL diffuses in the agar to reach the receiver cells containing LuxR which in turn triggers the hydrolase expression in the receiver colonies. The receiver cells comprise promoters that are tuned to express specific hydrolases depending on the amount of incoming AHL. The AHL diffusion is very important in that it drives the enzyme (hydrolase) expression in the non-mines depending on different high pass filters. The AHL concentration processed by the receiver cells depends on the number of mine colonies. Through an enzyme-susbtrate reaction that generates a colored product the player obtains information about the number of mines surrounding a non-mine.
Diffusion tests using AHL and a spiral receiver cell set-up
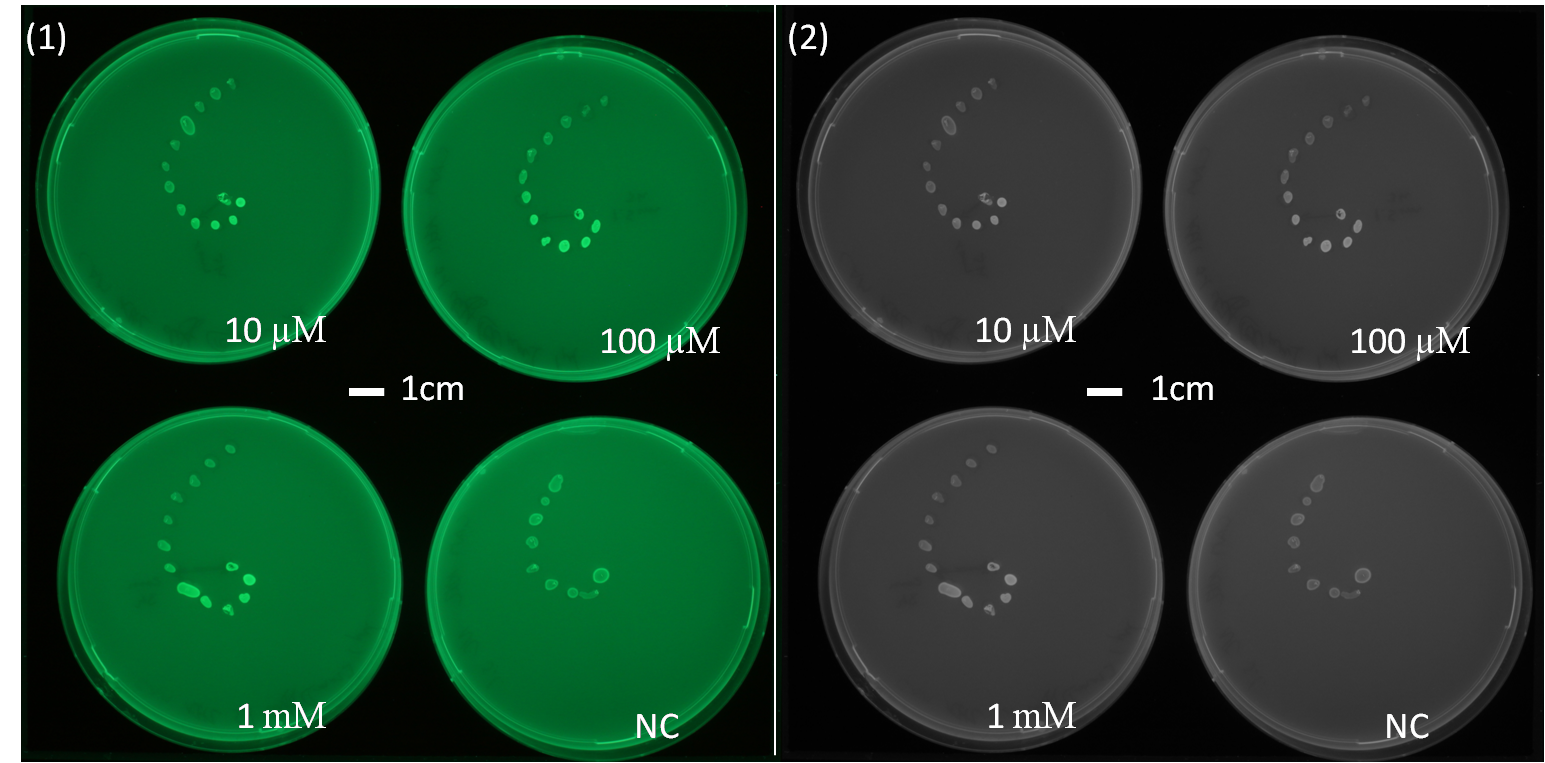
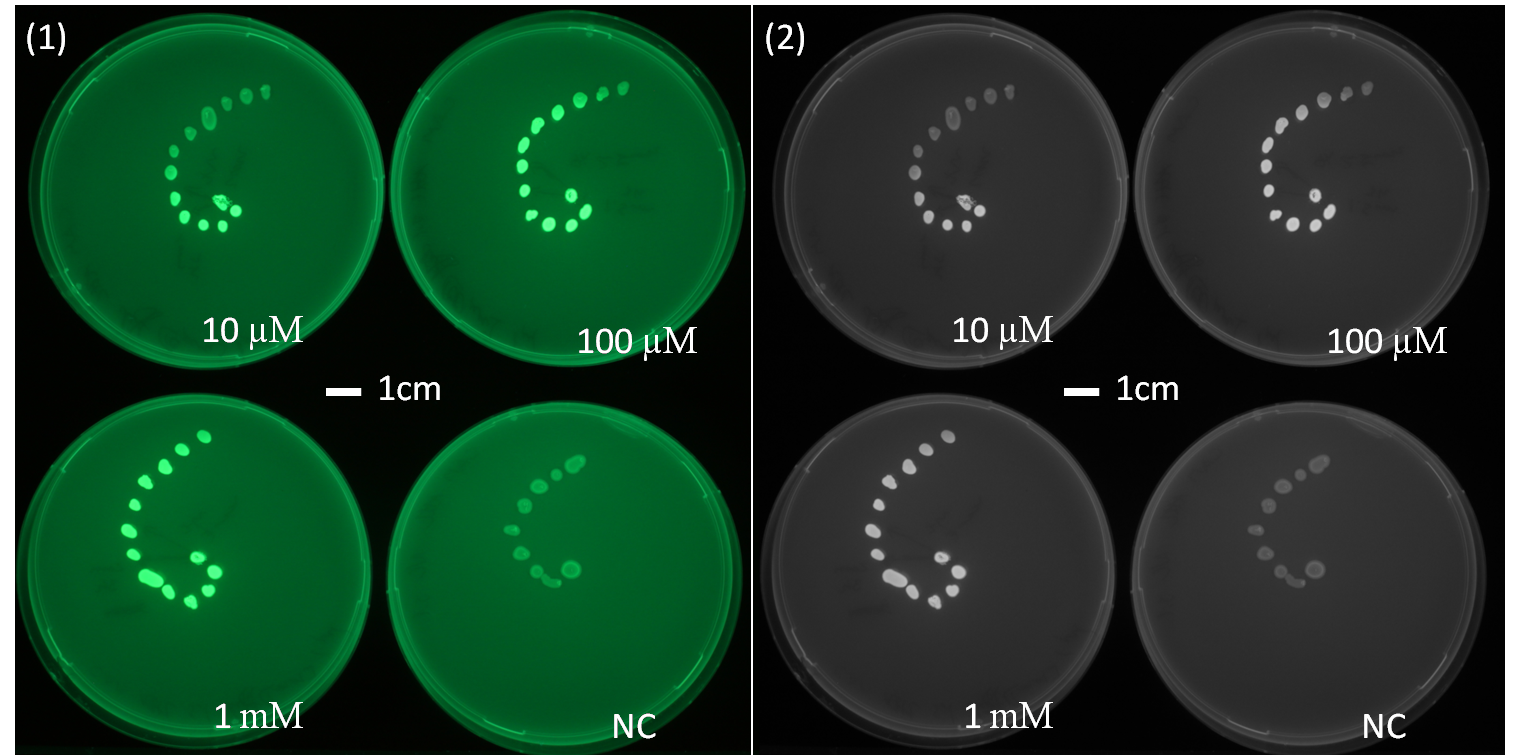
The experimental data of Figure 2.1 and 2.2 show a diffusion test using receiver cells ([http://parts.igem.org/Part:BBa_J09855 J09855] (Plac LuxR PluxR) with a GFP reporter) plated in a spiral pattern. The origin of diffusion: a drop of AHL with the according concentration was pipetted on the central colony. The experiment shows the diffusion over time, Figure 2.1 after 5 h of incubation and Figure 2.2 after 23 h of incubation at 37°C. The progression of the AHL throught the agar became visible thanks to the GFP reporter. This was one of the first experiments to characterize the diffusion of the signaling molecule in the agar as we need it later in the final set-up.(If you want to know more about the methods please click here.) We could conclude some information about the diffusion speed and about the distance of AHL diffusion. But we don't know about the AHL concentration secreted by a sender cell.
The next step was to carry out the same experiment using a sender colony instead of a drop of AHL in their origin of diffusion. (see below Diffusion tests using sender-receiver set-up).
Diffusion tests using sender-receiver set-up
In our biological circuit design, the sender secretes signalling molecule AHL. This molecule diffuses in and out of cells and thereby communicates between cells. As the AHL molecule diffuses it enters the neighboring non-mine cells. The non-mine cells are equipped with promoters that have different AHL sensitivities. This communication between mines and non-mines is vital in order to express the different output hydrolases. This is because the hydrolases are expressed under the control of PLuxR promoters which are induced by AHL that are diffused from the mines. Hence diffusion tests were performed to test diffusion time and distance of AHL from the sender to the receiver.
Diffusion tests of AHL in double layer agar
In order to visualize the AHL diffusion, experiments were carried out, using GFP as reporter. We started with using the part [http://parts.igem.org/Part:BBa_J09855 BBa_J09855] cloned with GFP as our receiver. We tested the sender with part [http://parts.igem.org/Part:BBa_K805016 BBa_K805016] under a constitutive promoter. double-layer agar tests (See protocol) were performed with the GFP receiver cells spread evenly as a top layer agar, and the 1.5 μl sender cells as source of signal AHL. The diffusion pattern was measured over 12 hours with a molecular imaging system. The distance of diffusion was noted as 1.5 cm as the radial diffusion distance across the sender colony. The time and distance data from these experiments were used for the spatio-temporal model of the AHL diffusion.
The image to the left shows the GFP fluorescence in a double layer agar experiment after 11 hours. In order to quantify the time and distance of diffusion of AHL from the sender to the GFP receiver, we analyzed the scanned image of the plate with the image processing program ImageJ . The analysis from ImageJ is shown in the picture to the right. The picture shows the GFP fluorescence in gray scale with distance in inches. The distance of diffusion can be seen as 1,2 inches, that is nearly 3 cm. Hence, for the further experiments, the colonies were placed apart from each other at a distance of 1.5 cm in a hexagonal manner.
 "
"