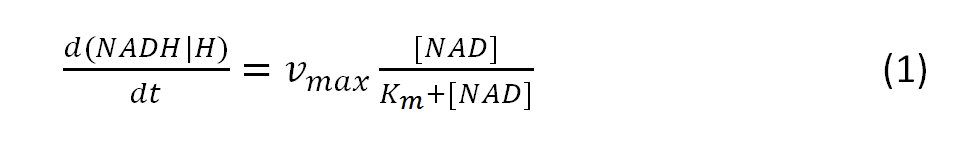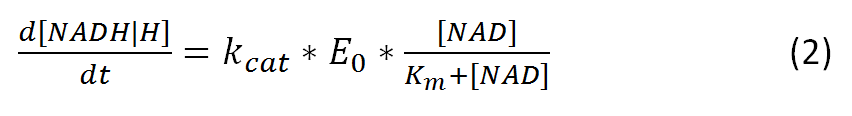Team:Bielefeld-Germany/Modelling/Inter
From 2013.igem.org
m |
m |
||
| Line 104: | Line 104: | ||
The values ''k<sub>cat</sub>'' and ''K<sub>m</sub>'' obtained as mentioned above were applied in the simulation of the | The values ''k<sub>cat</sub>'' and ''K<sub>m</sub>'' obtained as mentioned above were applied in the simulation of the | ||
production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec. | production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec. | ||
| - | The simulation was performed for five different NAD<sup>+</sup> concentrations: 10 µM, | + | The simulation was performed for five different NAD<sup>+</sup> concentrations: 10 µM, 50 µM, 100 µM and 500 µM . |
The MATLAB source code can be obtained as .m file [https://static.igem.org/mediawiki/2013/5/56/Bielefeld-germany-model-inter-matlab-code.m here]. | The MATLAB source code can be obtained as .m file [https://static.igem.org/mediawiki/2013/5/56/Bielefeld-germany-model-inter-matlab-code.m here]. | ||
| - | Exemplary curves of the concentration change over time for the start NAD<sup>+</sup> concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the | + | Exemplary curves of the concentration change over time for the start NAD<sup>+</sup> concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the product NADH for all simulated start concentration of substrate are shown in the Figure 3. |
<br><br> | <br><br> | ||
[[File:Bielefeld-germany-model-inter-diag-2.jpg|500px|center|thumb|'''Figure 1''': The curve of concentration change for the substrate NAD<sup>+</sup> and product NADH . Start concentration of substrate set at 10 µM]] | [[File:Bielefeld-germany-model-inter-diag-2.jpg|500px|center|thumb|'''Figure 1''': The curve of concentration change for the substrate NAD<sup>+</sup> and product NADH . Start concentration of substrate set at 10 µM]] | ||
| Line 113: | Line 113: | ||
<br> | <br> | ||
| - | |||
| - | |||
[[File:Bielefeld-germany-model-inter-diag-all2.PNG|500px|center|thumb|'''Figure 3''': The curve of concentration change of the product NADH within 10 seconds for different substrate start concentrations [NAD].]] | [[File:Bielefeld-germany-model-inter-diag-all2.PNG|500px|center|thumb|'''Figure 3''': The curve of concentration change of the product NADH within 10 seconds for different substrate start concentrations [NAD].]] | ||
<br><br> | <br><br> | ||
Latest revision as of 03:28, 29 October 2013
Modelling - Intermediates
Intermediates
The electrons that will be eventually transferred to the anode are generated during the biochemical processes within the bacterial cell. The central pathway in E.coli is the glycolysis and one of its key enzymes is the [http://www.genome.jp/dbget-bin/www_bget?ec:1.2.1.12 Glyceraldehyde 3-phosphate dehydrogenase (GAP-DH)]. As part of this reaction NAD+ is reduced to NADH+H+. Thus this intermediate takes part in the electron flow needed for the generation of electricity.
The resulting amount of the intermediate NADH+H+ can be calculated with the modeled Michaelis-Menten equation:
where vmax is the maximum rate achieved by the system
and Km describes the substrate concentration at which reaction rate is half-maximal.
Further vmax can be also represented as:
where kcat represents the rate-limiting turnover number
and E0 represents the enzyme concentration.
The resulting equation, alternative to (1), would be:
The data of the kinetics were obtained from the internet database [http://www.brenda-enzymes.org/php/result_flat.php4?ecno=1.2.1.12 BRENDA]. For E.coli the Km value for the NAD as a substrate is 0.045 for the wild-type enzyme. The kcat value for the E.coli for the wild-type is 0.268[1/s] ([http://abbs.oxfordjournals.org/content/44/6/527.long1 Errafiy, Soukri , 2012]).
Simulation
The values kcat and Km obtained as mentioned above were applied in the simulation of the
production of NADH. The start concentration of the enzyme GAP-DH was set at 10 µM and the simulation time span at 10 sec.
The simulation was performed for five different NAD+ concentrations: 10 µM, 50 µM, 100 µM and 500 µM .
The MATLAB source code can be obtained as .m file here.
Exemplary curves of the concentration change over time for the start NAD+ concentration of 10 µM and 1 M are shown in figure 1 and figure 2 respectively. The curves showing the concentration change of the product NADH for all simulated start concentration of substrate are shown in the Figure 3.
References
- Errafiy N, Soukri A (2012). Purification and partial characterization of glyceraldehyde-3-phosphate dehydrogenase from the ciliate Tetrahymena thermophila. [http://abbs.oxfordjournals.org/content/44/6/527.long Acta Biochimica et Biophysica Sinica, Volume 44](6), 527-534.
 "
"