Team:Paris Saclay/Notebook/July/8
From 2013.igem.org
Notebook : July 8
Summary:
FNR regulator system:
- performed another digestion for AmilCP, Pfnr plus sequence LacZ with Not I, Xho I, EcoR I and PST I.
- electrophoresis for those digest products
- enrichment culture for clone of the briobrick BBa_K1155000
- electrophoresis for extracted products of last friday
Lab work
Restriction digest
For some of the enzymes which are possible to use one common buffer, we did double digest
| DNA | enzyme | buffer |
| AmilCP1 | Not I | Orange |
| AmilCP2 | Not I | Orange |
| AmilCP1 | Xho I | Red |
| AmilCP2 | Xho I | Red |
| AmilCP1 | EcoR I | Orange |
| AmilCP2 | EcoR I | Orange |
| LacZ1 | EcoR I + PST I | Orange |
| LacZ2 | EcoR I + PST I | Orange |
| DNA | 2(µl) |
| buffer | 2(µl) |
| Enzyme(foreach) | 0.5(µl) |
| H20 | 15.5(µl) |
| total | 20(µl) |
The electrophoresis (135 V for 30 minutes) for digest products were migrated in agarose gel (0.5X).
Results:
|
Overnight incubation at 37°C with agitation.
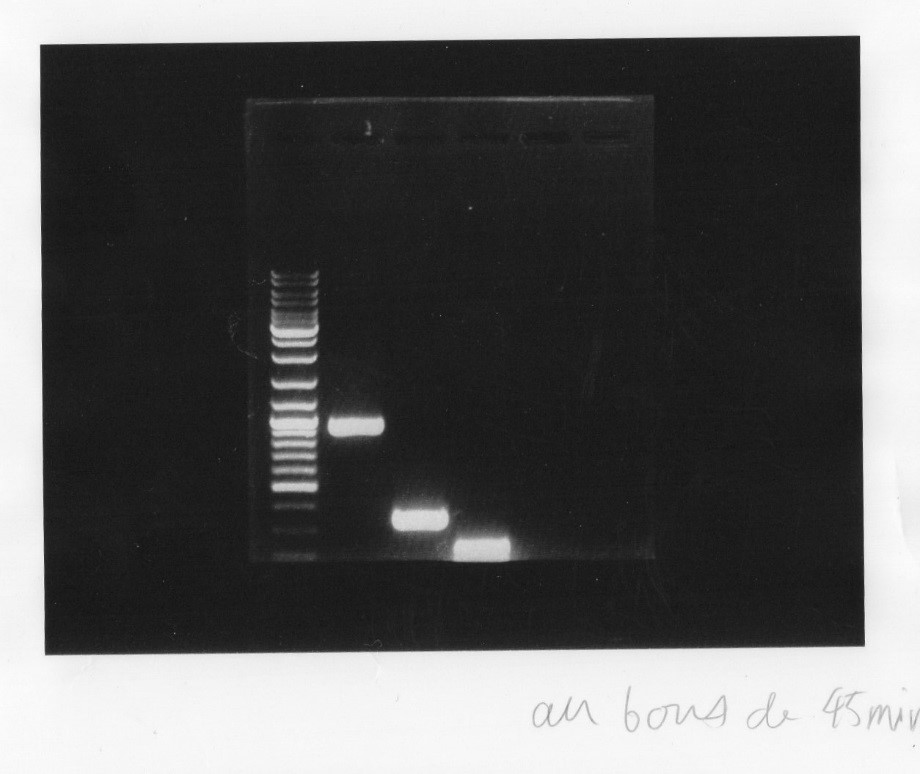
The electrophoresis for the plasmids which we had extracted last week plus 3 other BioBrick promoter Bph R1, promoter Bph A1, Bph R2.
Volume added:
- Pfnr2: 10µl
- R1P: 10µl
- R2: 10µl
- A1P: 10µl
Migration at 100V for 25mins then continued till 45 minutes:
Results:
|
The concentration of Pfnr2 was detected by NanoDrop :
- C = 1788.8 ng/µl
- 260/280 = 2.08
We considered that our extraction of plasmid DNA was not successful, we need to perform another extraction DNA.
Electrophoresis band size estimation
| Molecule | enzymes(Buffer) | Size |
| AmilCP clone | Nod I(Orange) | 2046+693bp |
| AmilCP clone | XHOI(Red) | 1842+892bp |
| BBa_K1155000 | XHOI(Red) | 1289+892bp |
| BBa_K1155000 | ECoR I(Orange) | 2151bp |
| Previous week | Back to calendar | Next day |
 "
"