Team:ETH Zurich/Optimization
From 2013.igem.org
Different attempts to reduce the leakiness
Optimization of LuxR production regulation to reduce basal reporter activation
The GFP reporter system shows significant leakiness in the absence of OHHL. To pinpoint the source of this basal activation we tested the GFP expression of different constructs in liquid culture over time (Figure 6). We defined the background using a construct without GFP (Figure 5, top left). We used a simple pLuxR-GFP construct without LuxR protein (Figure 5, top right) to measure the basal expression resulting from promoter leakiness alone. The measured signal is comparable to background levels, suggesting that pLuxR promoter per se is remarkably tight.
The complete pLac-Lux-pLuxR-GFP receiver construct (Figure 5, bottom left) in the absence of OHHL induction shows a jump in the measured GFP levels. This results suggest that most of the leakiness comes from an activation of the pLux promoter through LuxR alone, meaning in the absence of the OHHL inducer (Figure 3).
Addition of OHHL (Figure 5, bottom right) determines a steep 5-7 times GFP induction. Reduction of the LuxR dependent basal activation could significantly improve the On/Off Ratio.
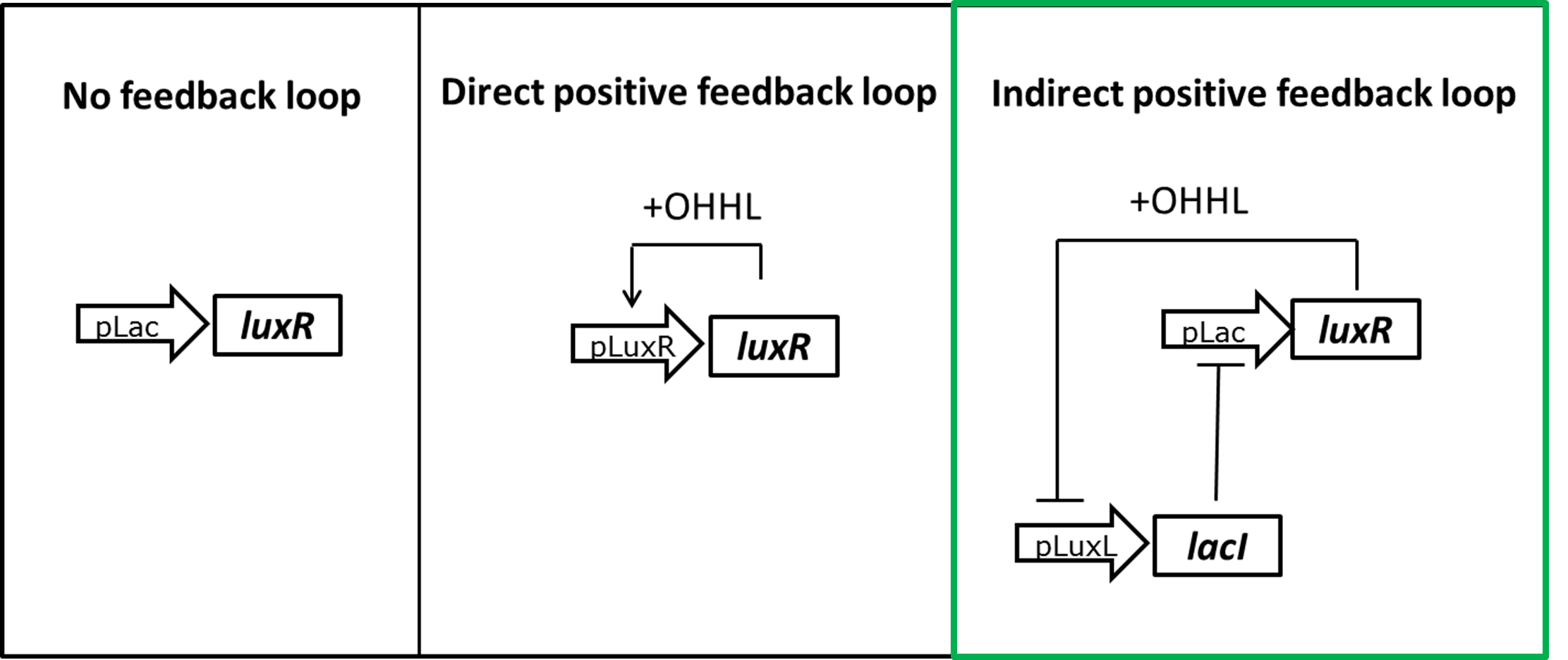
Hydrolase reporters are enzymatic systems. Multiple rounds of catalysis provide intrinsic signal amplification when compared to GFP. Therefore we expect a very high sensitivity to basal expression. Moderate hydrolase expression level could already result in total substrate conversion and signal saturation. Since our model anticipates the potential problem we designed different strategies to reduce the level of LuxR in the uninduced state (Figure 7).
The overall idea was to replace a constitutive LuxR expression with an OHHL dependent induction. In a self-regulation motif only a residual amount of LuxR is present in the non induced state, minimizing its interaction with the promoter. Upon induction the protein sustain its own production allowing for a full activation of the system. To achieve autoregulation one possibility is to use a direct positive feedback loop in which the luxR gene is placed under it's own pLuxR promoter. Only upon induction with OHHL LuxR would be produced. As an alternative we could use LacI expression to inhibit LuxR production in the uninduced state. By placing the lacI gene under a negatively LuxR/OHHL regulated promoter (pLuxL) an indirect positive autoregulation for LuxR expression is obtained.
 "
"