Team:ETH Zurich/Experiments 3
From 2013.igem.org
Contents[hide] |
Enzyme-substrate reactions
To generate visible output by adding substrates to colonies in Colisweeper, we made use of orthogonal enzyme-substrate reactions. A set of chromogenic substrates was chosen to produce different colors depending on the abundant hydrolases and thereby to uncover the identity of each colony to the player.
The set of enzyme-substrate pairs chosen for the Colisweeper project, and characterizations of their reactions, are described below. Information on other possible substrates that can be used for the enzymes of the Colisweeper reporter system can be found in the reporter system section.
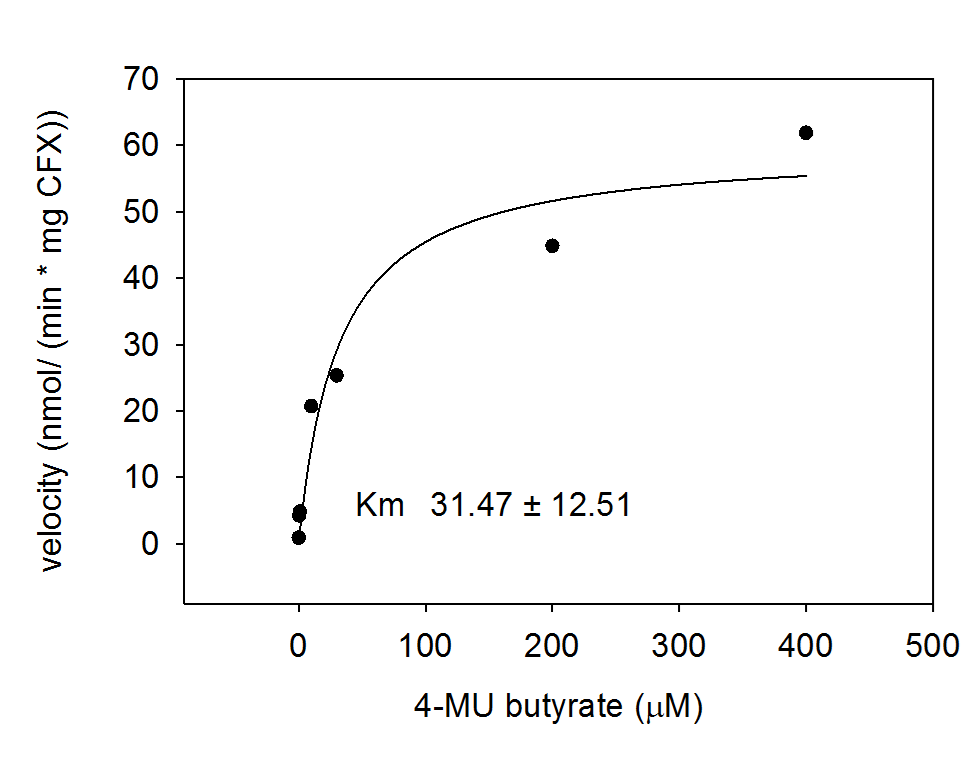
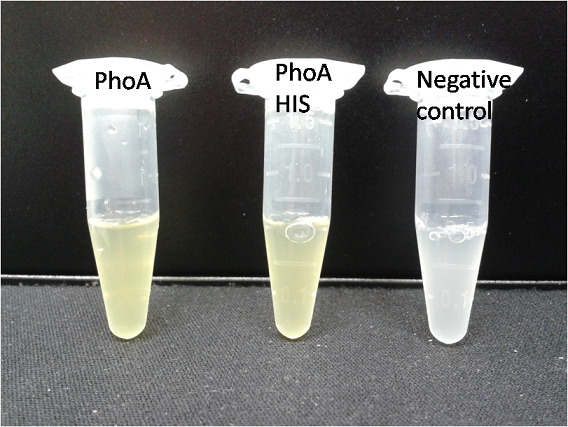
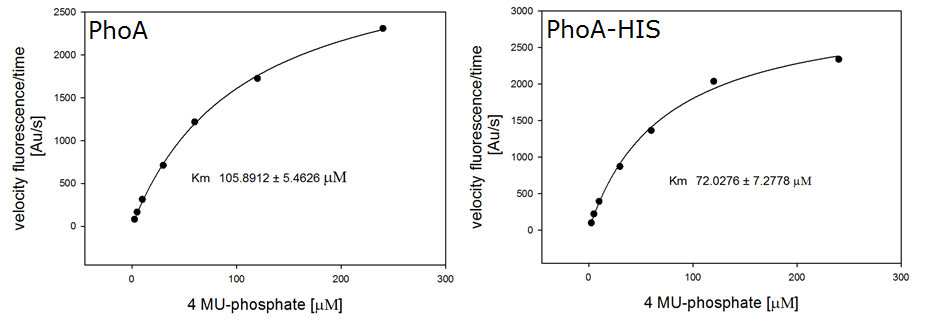
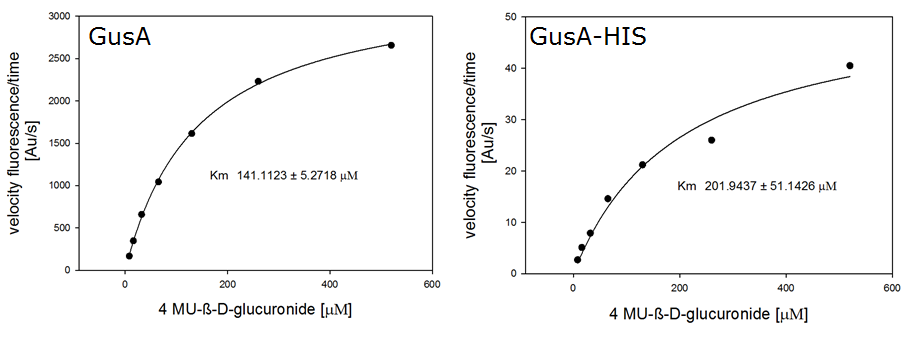
To characterize the hydrolases used in Colisweeper, we conducted substrate tests in liquid cultures as well as on colonies, enzyme kinetics and detection of HIS-tagged protein by Western Blot. Additionally, crosstalk assays and color overlay tests (multiple enzyme-substrate reactions together) have been performed for this project. A listing of chromogenic substrates used can be found in the Materials section.
General characterisitcs overview
| Substrate | Hydrolase | Color, Absorption λmax |
Stock | Liquid culture | Colonies | Response time on E. coli colonies, at RT |
|---|---|---|---|---|---|---|
| 5-Bromo-4-Chloro-3-indoxyl-β-D-galactopyranoside (X-Gal) | LacZ | Blue, 615 nm |
0.5 M in DMSO | 1 mM | 50 mM | ~ 10 minutes |
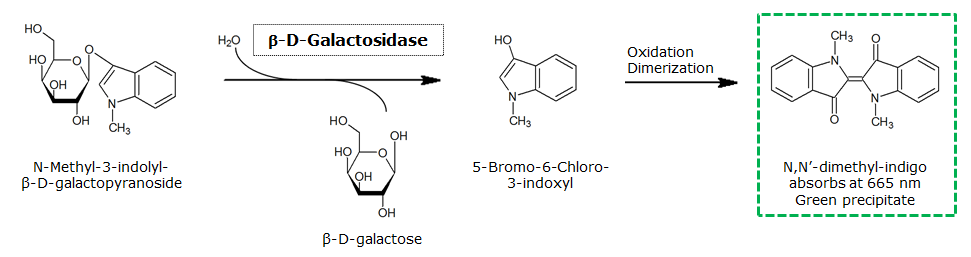
| N-Methyl-3-indolyl-β-D-galactopyranoside (Green-Gal) | LacZ | Green, 665 nm |
0.1 M in DMSO | 1 mM | 50 mM | ~ 15 minutes |
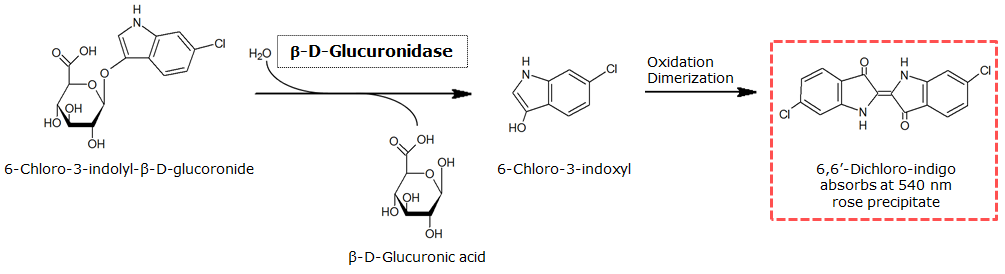
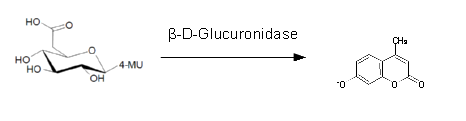
| 6-Chloro-3-indolyl-β-D-glucuronide (Salmon-Glc) | GusA | Salmon, 540 nm |
0.3 M in DMSO | 1.5 mM | 0.1 M | ~ 5 minutes |
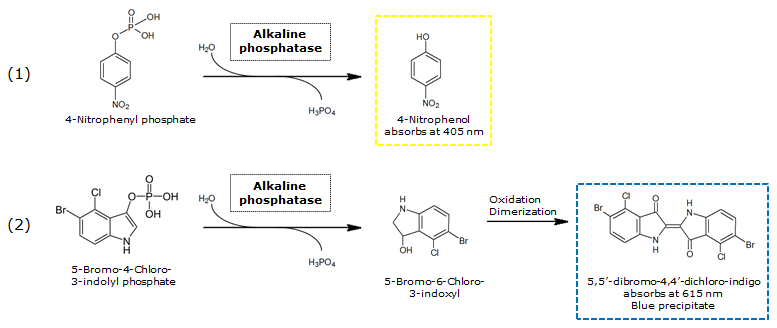
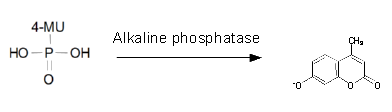
| 4-Nitrophenoyl-phosphate (pNPP) | PhoA | Yellow, 405 nm |
0.5 M in DEA | 50 mM | 0.5 M | ~ 1 minute |
| 5-Bromo-4-Chloro-3-indolyl phosphate (BCIP) | PhoA | Blue, 615 nm |
0.1 M in H2O | 1 mM | 50 mM | ~ 30 minutes |
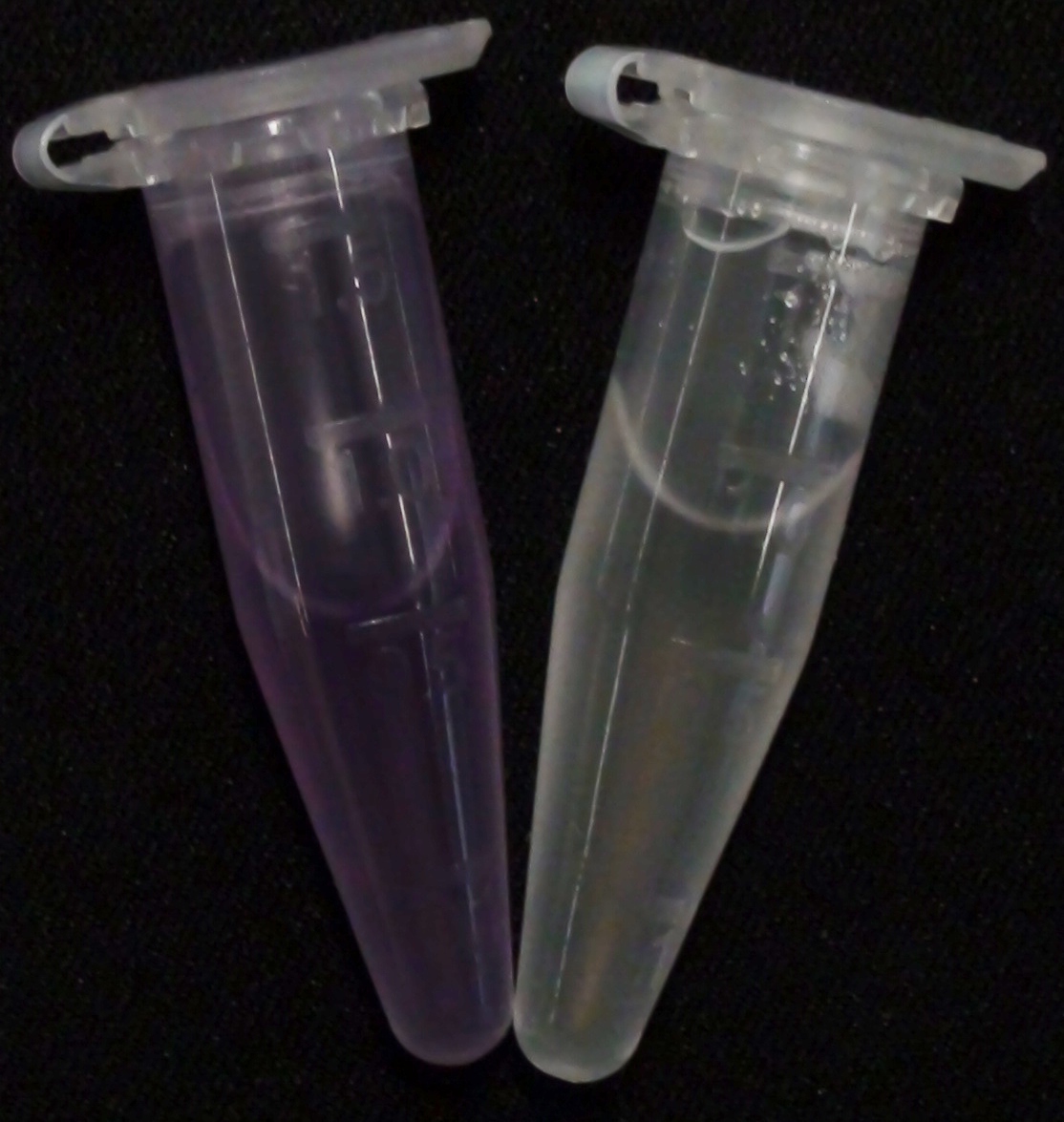
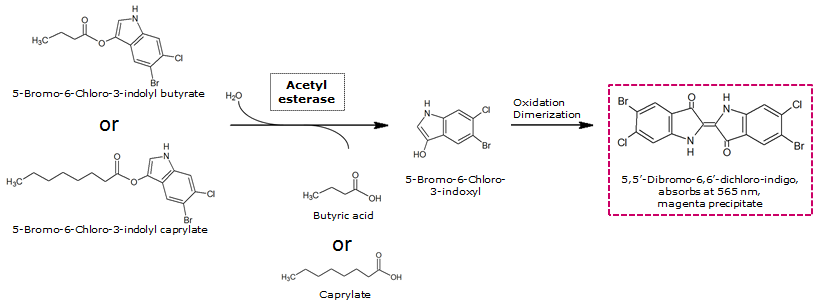
| 5-Bromo-6-Chloro-3-indoxyl butyrate (Magenta butyrate) | Aes | Magenta, 565 nm |
0.5 M in Acetone | 0.1 mM | 20 mM | ~ 2 minutes |
| 5-Bromo-6-Chloro-3-indoxyl caprylate (Magenta caprylate) | Aes | Magenta, 565 nm |
0.2 M in Acetone | 1 mM | 0.2 M | overnight |
| 4-Nitrophenyl- N-acetyl-β-D-glucosaminide (pNP-GluNAc) | NagZ | Yellow, 405 nm |
15 mM in H2O | 0.01 mM | 15 mM | ~ 1 minute |
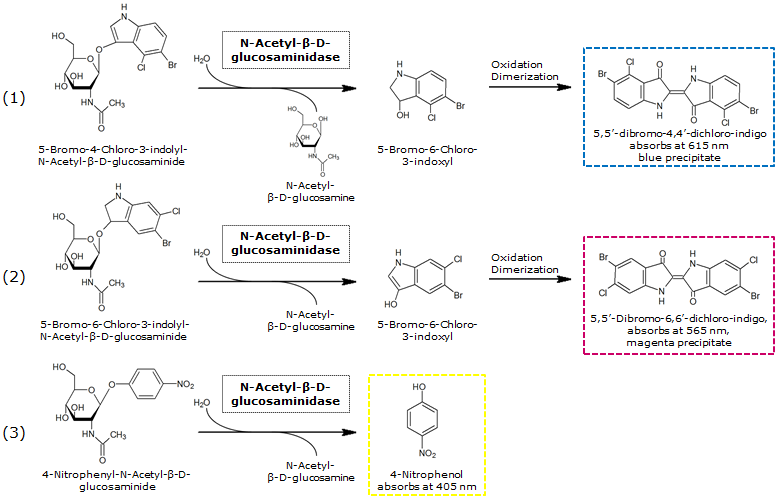
| 5-Bromo-6-Chloro-3-indolyl N-acetyl-β-D-glucosaminide (Magenta GluNAc) | NagZ | Magenta, 565 nm |
0.1 M in DMSO | 1 mM, supplemented with BSA | 50 mM, with BSA added | ~ 15 minutes |
| 5-Bromo-4-Chloro-3-indolyl N-acetyl-β-D-glucosaminide (X-GluNAc) | NagZ | Blue, 615 nm |
0.1 M in DMSO | 1 mM, supplemented with BSA | 50 mM, with BSA added | ~ 15 minutes |
Expand the boxes to see the characterization of the hydrolases
Acetyl esterase (Aes)
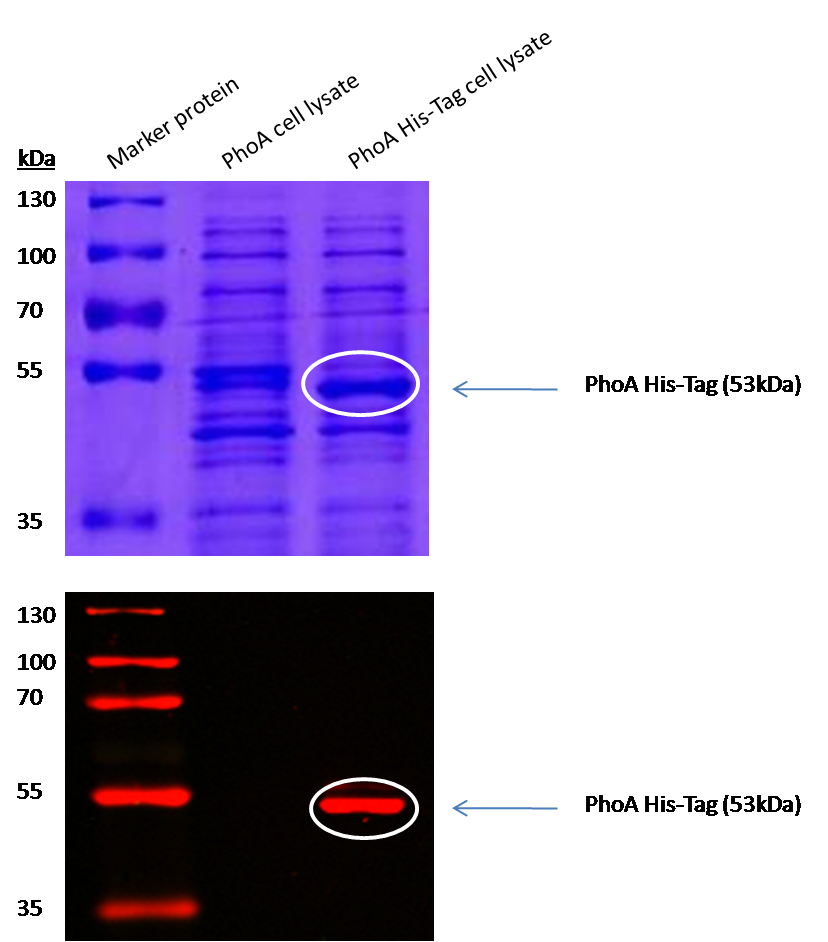
Alkaline phosphatase (PhoA)
β-Galactosidase (LacZ)
β-Glucuronidase (GusA)
β-N-Acetylglucosaminidase (NagZ)
Crosstalk
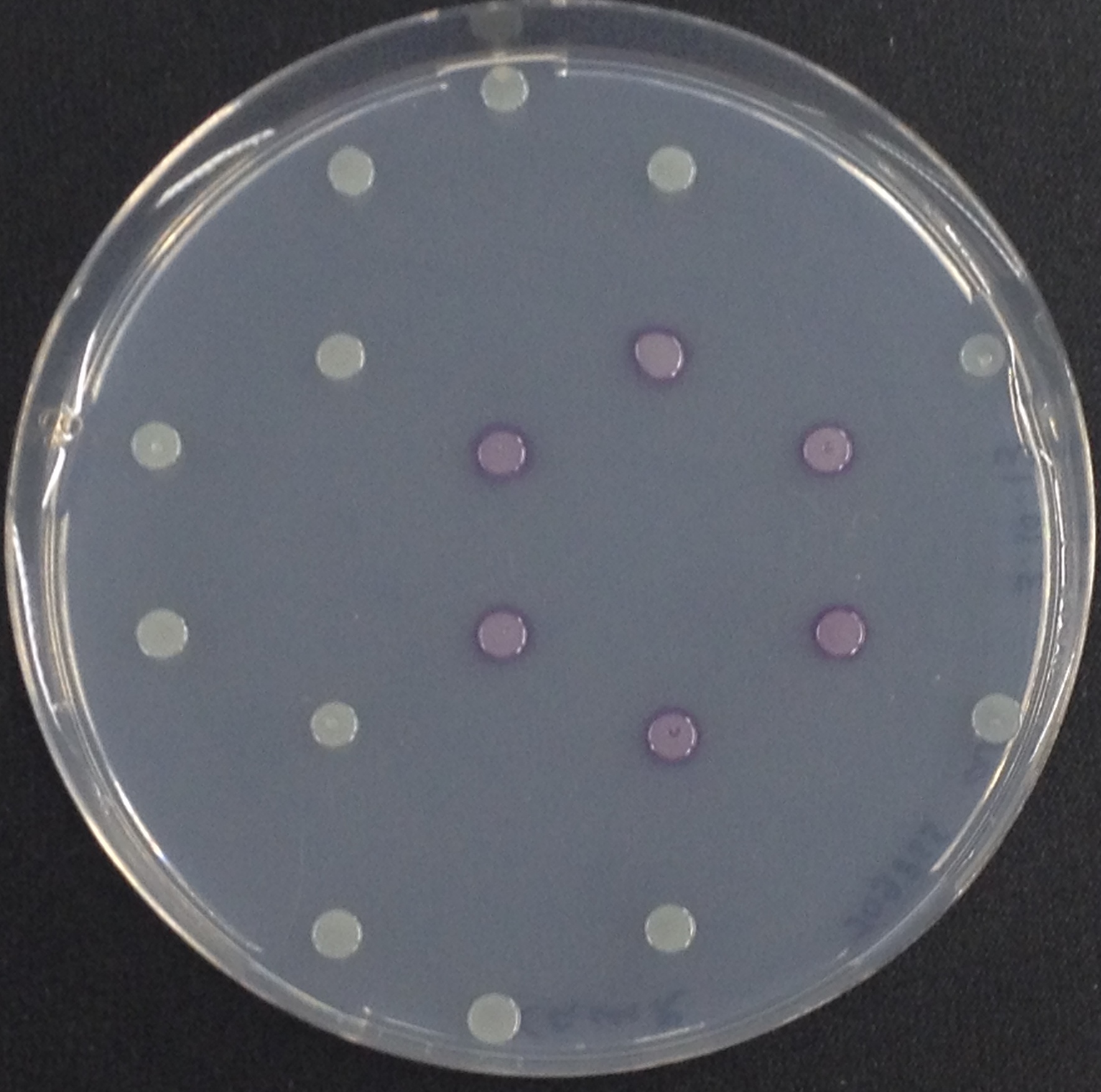
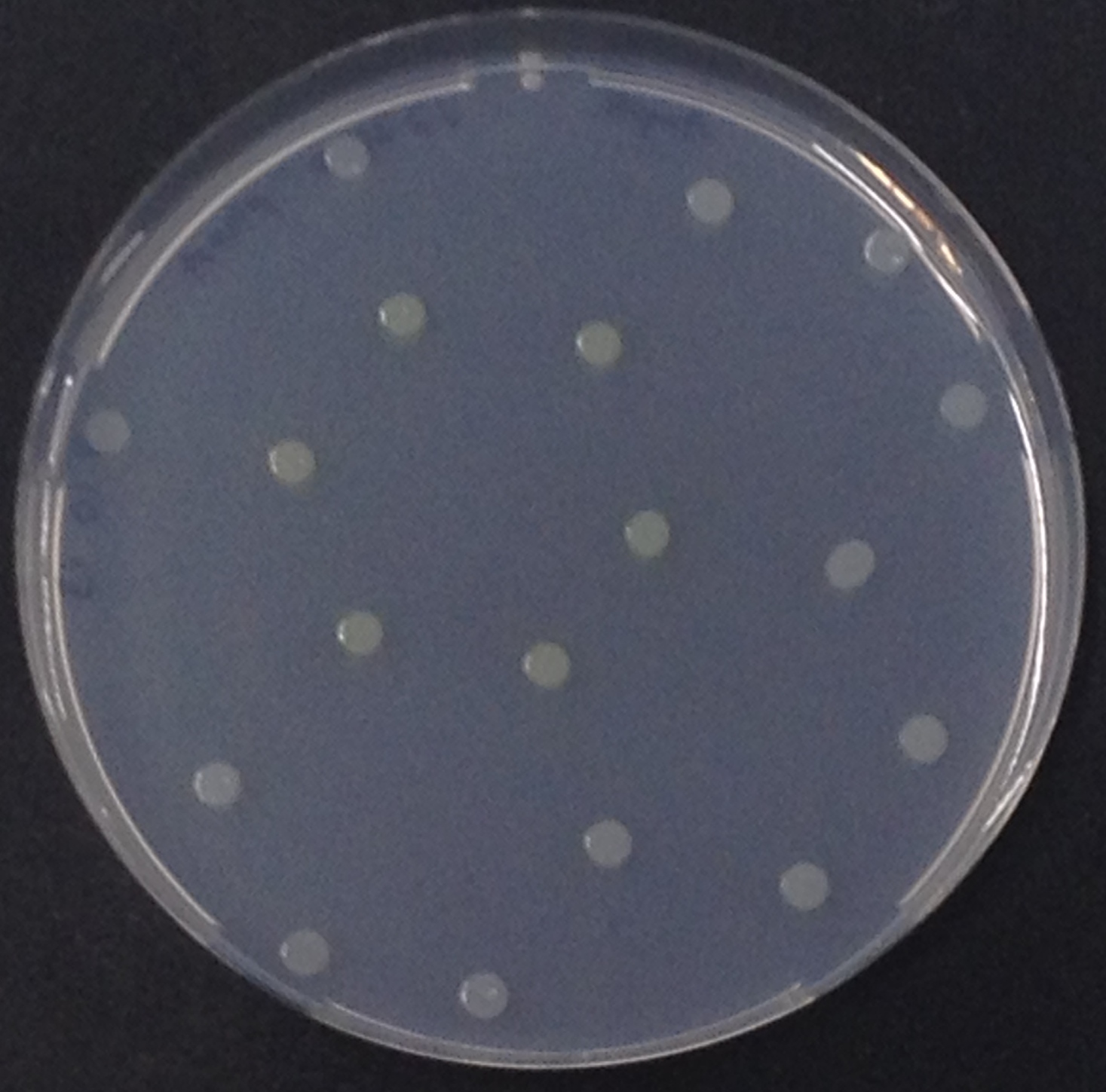
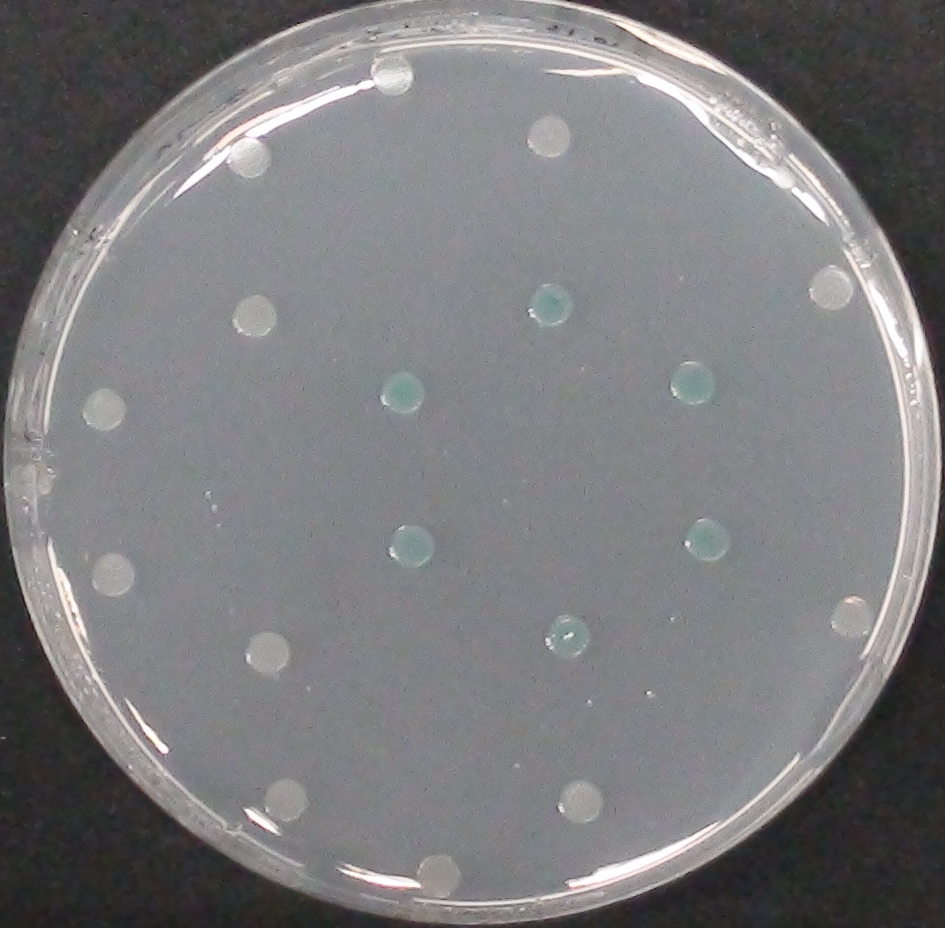
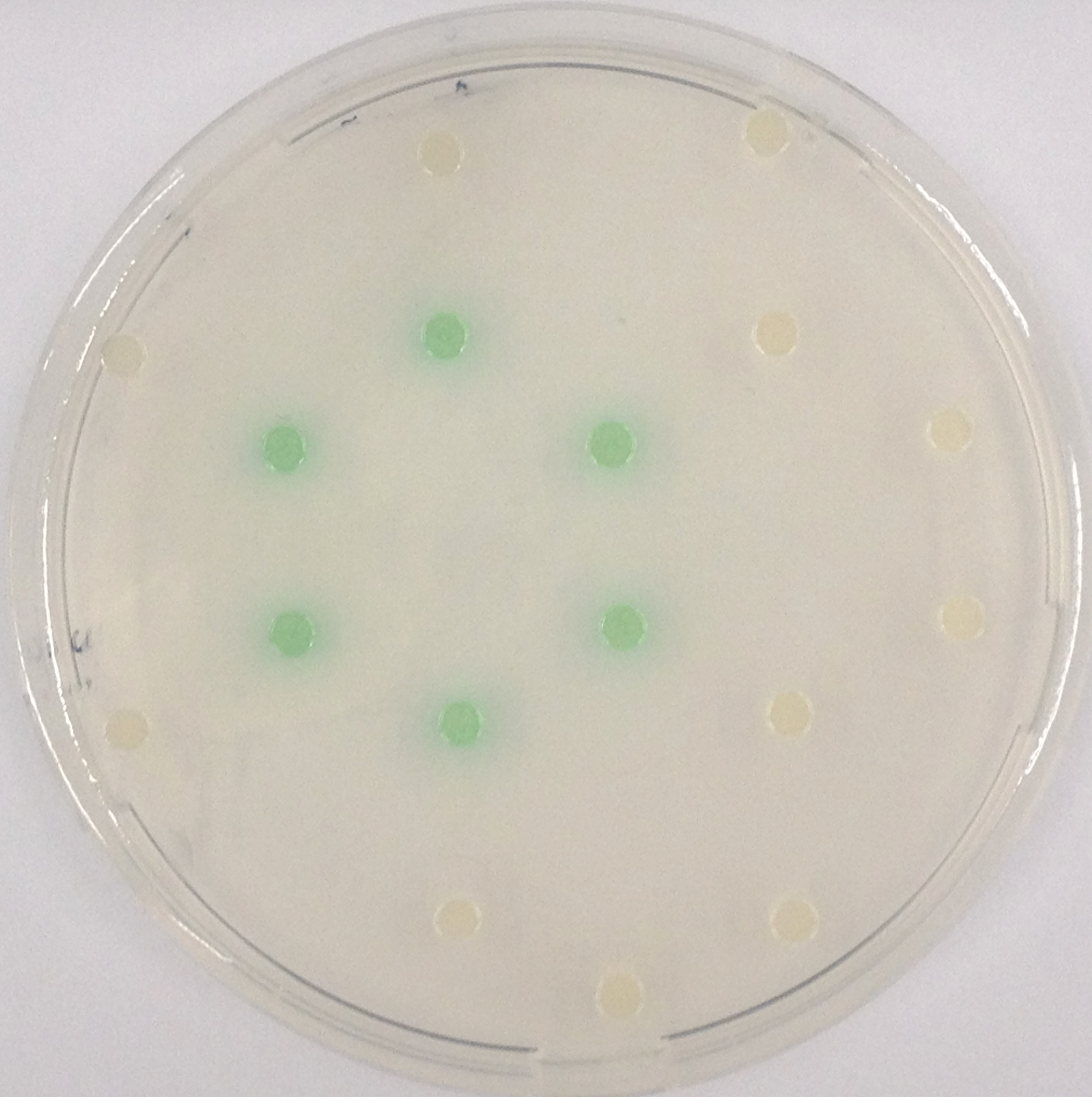
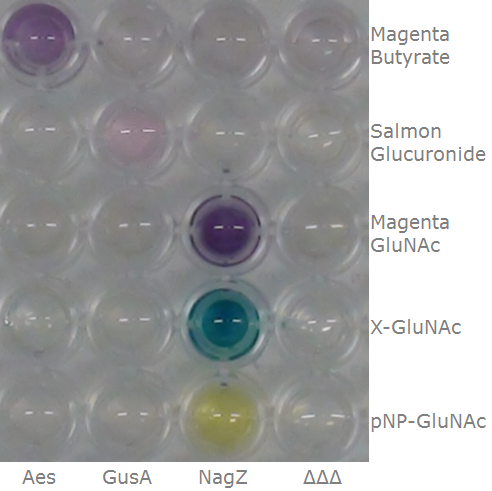
To ensure specificity of the enzyme-substrate pairs used in Colisweeper, a crosstalk test was done to make sure that all overexpressed enzymes specifically cleave their assigned substrate.
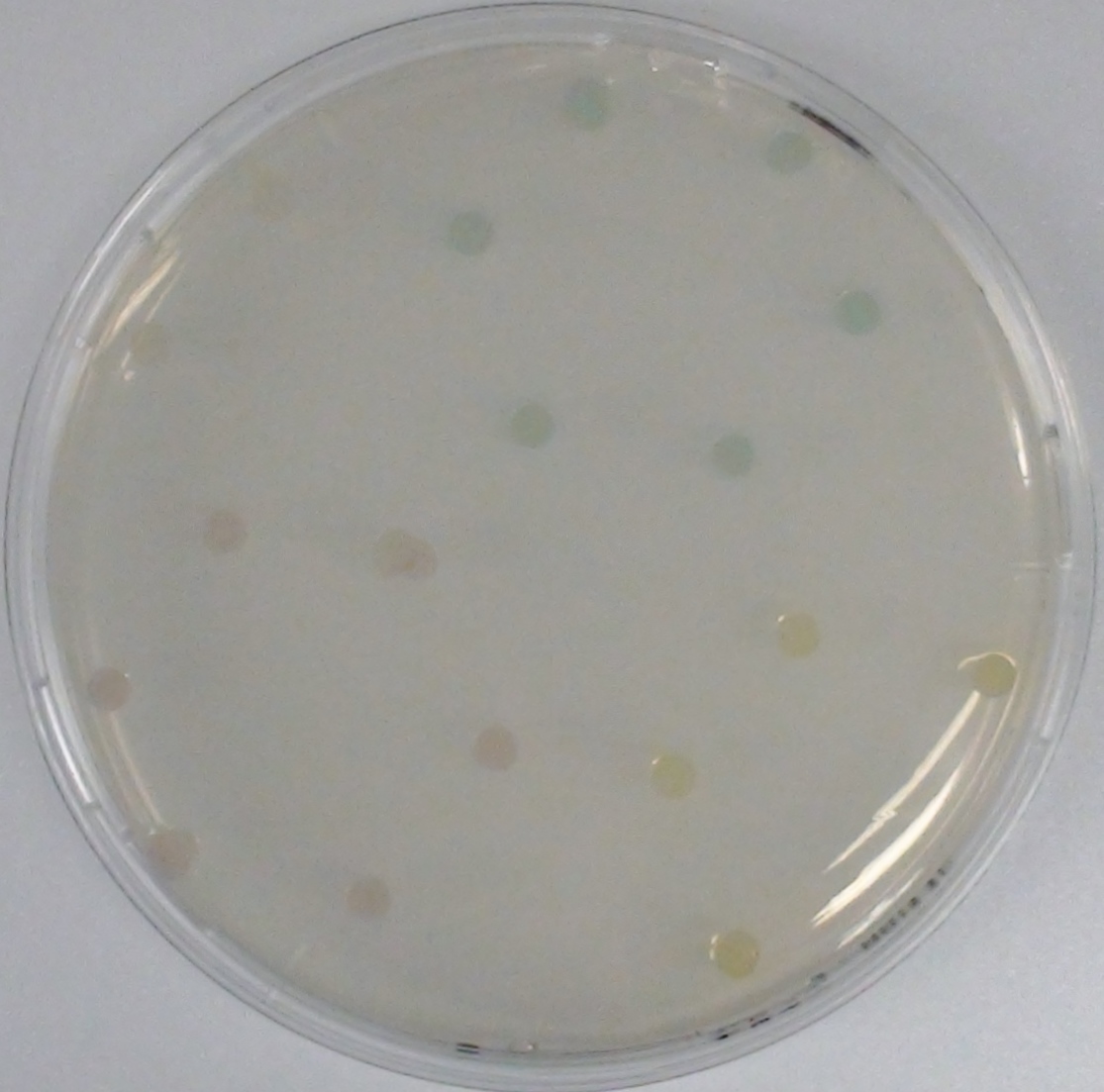
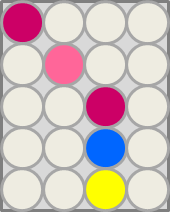
This crosstalk test was done in a 96-well plate, each well containing 200 μl from liquid cultures of our ΔaesΔgusAΔnagZ Escherichia coli strain overexpressing either Aes, GusA, NagZ or none, each distributed among the column-wells of the plate. Horizontally, the chromogenic substrates were pipetted to the liquid cultures in the same order as their corresponding hydrolase. If specificity of the chosen enzyme-substrates pairs were given, we would expect an output as shown in Figure 26.1.:
As Figure 26.2. shows, the overexpressed hydrolases cleave only the substrates they were expected to.
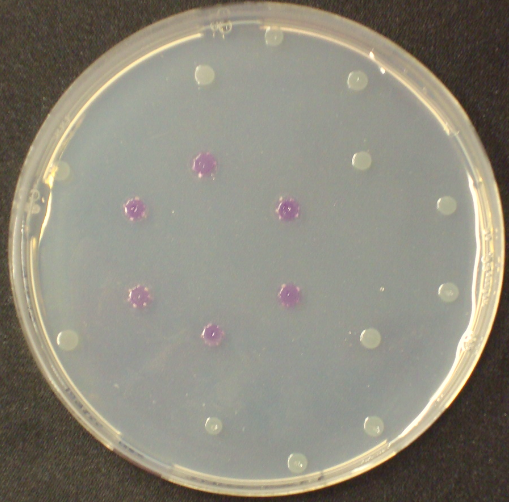
Color overlay
To play Colisweeper, colors for each colony identity have to be clearly distinguishable by eye. Because we are using high-pass filters to differentially process certain AHL levels, we had to make sure that a mix of cleaved product would show distinguishable colors.
References
(1) Orenga S, Roger-Dalbert C, James A, Perry J, US Patent 20090017481 (2009).
(2) Magnelli PE, Bielik A, Guthrie E, Methods Mol. Biol., 801, 189-211 (2011).
Absorption: Biosynth
 "
"