Team:ETH Zurich/Experiments 2
From 2013.igem.org
Contents |
Quorum sensing signaling molecule
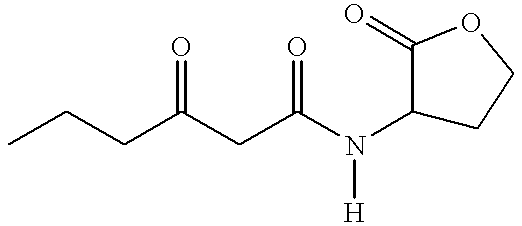
N-3-Oxo-Hexanoyl-l-Homoserine Lactone (OHHL) belongs to the family of Acylated Homoserine Lactones (AHL).
In our project , we use the LuxI-LuxR quorum sensing system to drive the signal from the sender to the receiver cells. The LuxI sender construct produces OHHL. The OHHL diffuses in the agar to reach the receiver cells containing LuxR which in turn triggers the hydrolase expression in the non-mine colonies. The receiver cells comprise promoters that are tuned to express specific hydrolases depending on the amount of incoming OHHL. The OHHL diffusion is very important in that it drives the enzyme (hydrolase) expression in the non-mines depending on different high pass filters.The OHHL concentration of course depends on the number of mine surrounding receiver colonies. Through an enzyme-susbtrate reaction that generates a colored product the player obtains information about the number of mines surrounding a non-mine.
Diffusion tests of OHHL
As part of our system design, the diffusion of OHHL from the sender to the receiver is vital in order to express the different orthogonal hydrolases. To the end of visualizing the diffusion, experiments were carried out, using GFP as reporter, to characterize speed and distance of OHHL diffusion. The data from these experiments were used for the spatio-temporal model of the OHHL diffusion.
Double layer agar experiments were carried out to check diffusion of different concentrations of the OHHL. On a normal 1.5% agar plate, a second thinner layer of 0.7% agar, inoculated with 100μl receiver cells, is spread to form the upper agar layer. A 1.5μl drop of OHHL was added on the middle of the plate and the diffusion was observed until eleven hours.
Single layer agar grid diffusion tests with sender-receiver
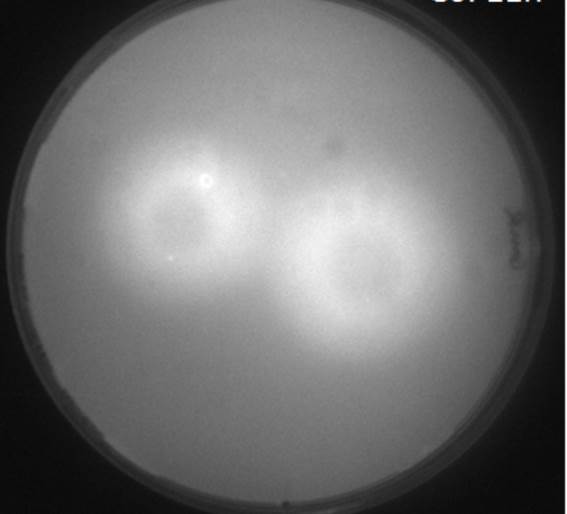
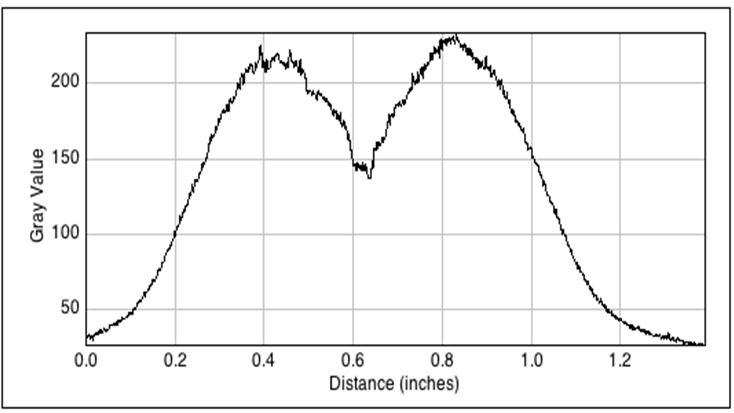
In single layer agar diffusion experiments we tested the effects of diffusion from the sender cells to the receiver cells with the placement in different possible configurations. Different configurations of mines around the non-mines in the sense of number of mines around a non-mine will lead to more OHHL. These results were then used for the spatio-temporal model of OHHL diffusion. We then observed the diffusion patterns from fluorescent images and grey-scale images. It was noted that the background noise was relatively high and the two higher concentrations diffuse to similar distances after 11 hours of incubation.
Diffusion experiments were carried out with the sender-receiver constructs as well. With the hexagonal grid, we plated out 1.5μl of the sender-receiver cells from the log phase liquid cultures on a 1.5% gar plate. After incubaion for three hours, the diffusion of OHHL was observed for over a period of 11 hours until saturation of the GFP expression was observed in the receiver cells
GFP diffusion with mutated pluxR promoter variants
See first : pluxR Mutations
In order to test the GFP fluorescence pluxR promoters that were mutated by site-saturation mutagenesis , two layer agar experiments were performed with mutated and wildtype promoters. In every case, the expression was compared with a negative control without the sender (pconst-luxI) which will not induce the GFP expression. We followed the grid pattern with the different configurations of mines in a hexagonal pattern.The figure to the left shows the expression in the wildtype pluxR with the negative controls to the right of the picture. The figure to the right shows the expression in the mutated promoter with two point mutations in the sequence. The negative controls without the sender cells are to the right of the picture.
 "
"