Team:ETH Zurich/Modeling
From 2013.igem.org
| Line 32: | Line 32: | ||
<p align="justify"> Two processes take place on the agar: diffusion and decay of AHL. The equation for AHL in agar plate is given below: </p> | <p align="justify"> Two processes take place on the agar: diffusion and decay of AHL. The equation for AHL in agar plate is given below: </p> | ||
\begin{align} | \begin{align} | ||
| - | \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL-d_{AHL,e} \cdot [AHL] | + | \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL-d_{AHL,e} \cdot [AHL]\\ |
| - | \end{ | + | \end{align} |
<h1>Receiver Cells</h1> | <h1>Receiver Cells</h1> | ||
| - | <p align="justify">Receivers are engineered to respond differently to two | + | <p align="justify">Receivers are engineered to respond differently to two AHL concentration levels. Basically, cells should be capable of produce a visible response and in a reasonable amount of time, because the player will need to discriminate between the presence of 0, 1 or 2 adjacent mines. To achieve this goal, we incorporate two enzymatic reporters acting as [https://2013.igem.org/Team:ETH_Zurich/Experiments_5high-pass filters] ([https://2013.igem.org/Team:ETH_Zurich/Experiments_7 GusA] and [https://2013.igem.org/Team:ETH_Zurich/Experiments_7 AES]). The expression of these enzymes is under the control of ''pLux'' promoters with different sensitivities, the wild type and a promoter mutant ([https://2013.igem.org/Team:ETH_Zurich/Experiments_5 G1 mutant]); The affinity constants for the promoters were obtained from our [https://2013.igem.org/Team:ETH_Zurich/Experiments_5 experimental data]. An importan feature of these enzymes is that they can catalyze the hydrolysis of various chromogenic compounds to give rise to a relatively quick coloured response. </p> |
<br> | <br> | ||
| - | The intracellular species of interest in the receiver cells module include: LuxR, | + | The intracellular species of interest in the receiver cells module include: LuxR, AHL, LuxR/AHL complex (denoted as R) and the hydrolases (GusA and AES). |
| - | + | ||
| - | + | ||
| + | \begin{align} | ||
| + | \frac{d[LuxR]}{dt}=\alpha_{[LuxR]} - d_{LuxR} [LuxR] \\ | ||
| + | \end{align} | ||
| + | \begin{align} | ||
| + | \frac{d[R]}{dt}=\rho_{LuxR} \cdot [LuxR ]^2 \cdot [AHL]^2 - d_{R} [R]\\ | ||
| + | \end{align} | ||
| + | \begin{align} | ||
| + | \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL - DF \cdot d_{AHL} \cdot [AHL] \\ | ||
| + | \end{align} | ||
| + | \begin{align} | ||
| + | \frac{d[GusA]}{dt}=\alpha_{GusA} \cdot k_{GusA} + \frac{\alpha_{GusA}(\frac{R}{K_{R_1}})^{n_{1}}}{1+(\frac{R}{K_{R_1}})^{n_{1}}} - d_{GusA}\cdot [GusA]\\ | ||
| + | \end{align} | ||
| + | \begin{align} | ||
| + | \frac{d[AES]}{dt}=\alpha_{AES} \cdot k_{AES} + \frac{\alpha_{AES}(\frac{R}{K_{R_2}})^{n_{2}}}{1+(\frac{R}{K_{R_2}})^{n_{2}}} - d_{AES}\cdot [AES]\\ | ||
| + | \end{align} | ||
<p align="justify"> In addition to new proteins incorporated to the circuit, it is important to emphasize that the grid was changed to a three neighbours setup.</p> | <p align="justify"> In addition to new proteins incorporated to the circuit, it is important to emphasize that the grid was changed to a three neighbours setup.</p> | ||
<br clear="all"/> | <br clear="all"/> | ||
| Line 49: | Line 62: | ||
<p align="justify"> | <p align="justify"> | ||
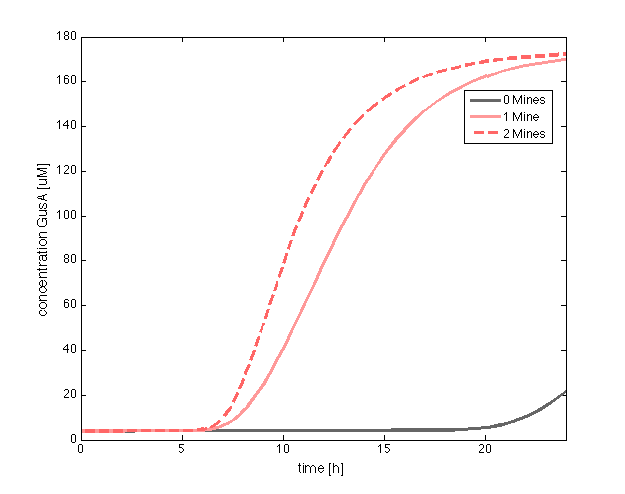
| - | In figure 1 and 3 are shown the results regarding GusA expression levels. From the time course plot (Fig 1.), it can be pointed out that the activation of the gene is consistent with the | + | In figure 1 and 3 are shown the results regarding GusA expression levels. From the time course plot (Fig 1.), it can be pointed out that the activation of the gene is consistent with the AHL concentration that is predicted to reach a receiver colony. Additionally, one can say that the incubations of the plates should not be longer than 17 hours, otherwise receiver cells that are more that one cell away from a mine can start synthesizing the enzyme. </p> |
<br> | <br> | ||
{|style="border: none;" align="center" | {|style="border: none;" align="center" | ||
|valign="top"|[[File:GusA.png|500px|center|thumb|<b>Figure 1: Averaged GusA concentration </b> for a colony with 0, 1 or 2 adjacent mines cells, respectively. Distance between colonies: 1.5 cm. ]] | |valign="top"|[[File:GusA.png|500px|center|thumb|<b>Figure 1: Averaged GusA concentration </b> for a colony with 0, 1 or 2 adjacent mines cells, respectively. Distance between colonies: 1.5 cm. ]] | ||
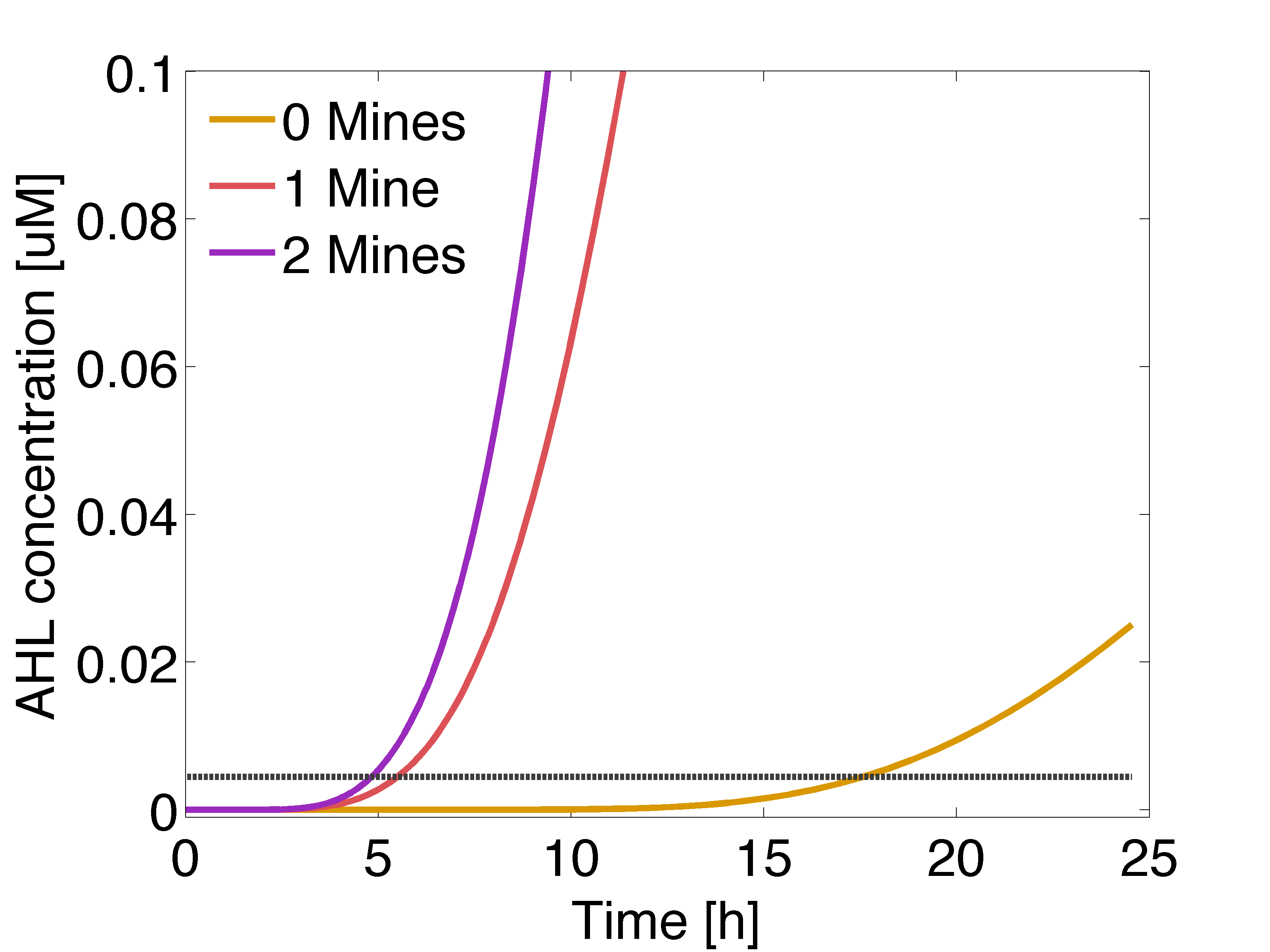
| - | |valign="top"|[[File:Ahl gusA.png|500px|center|thumb|<b>Figure 2: Averaged | + | |valign="top"|[[File:Ahl gusA.png|500px|center|thumb|<b>Figure 2: Averaged AHL concentration </b> sensed by a colony with 0, 1 or 2 adjacent mines cells, respectively. The horizontal line indicates the activation coefficient by the dimer LuxR/AHL (K<sub>R1</sub>). Distance between colonies: 1.5 cm.]] |
|} | |} | ||
<br> | <br> | ||
{|style="border: none;" align="center" | {|style="border: none;" align="center" | ||
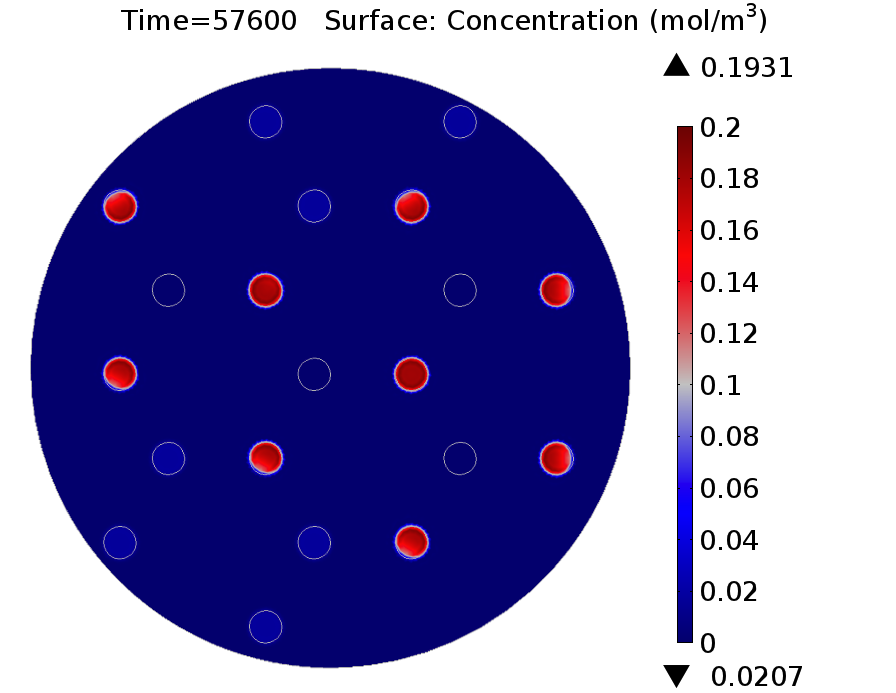
|valign="top"|[[File:GusA 16h.png|500px|center|thumb|<b>Figure 3: GusA expression profile </b> after 16 hours. Display: GusA concentration in mM. Distance between colonies: 1.5 cm.]] | |valign="top"|[[File:GusA 16h.png|500px|center|thumb|<b>Figure 3: GusA expression profile </b> after 16 hours. Display: GusA concentration in mM. Distance between colonies: 1.5 cm.]] | ||
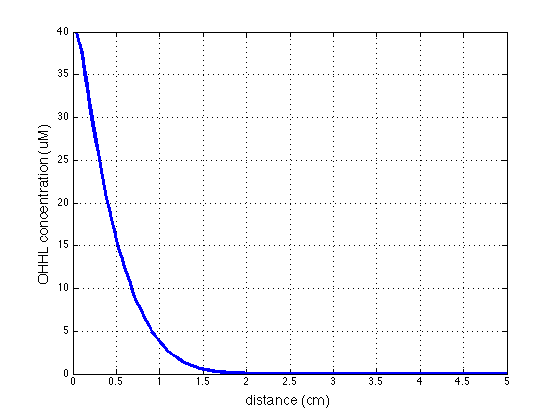
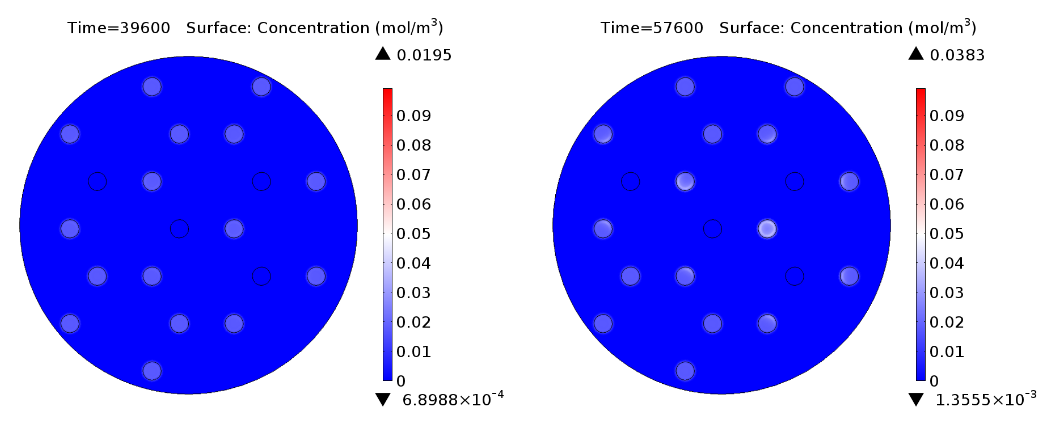
| - | |valign="top"|[[File:AHL 16h.png|500px|center|thumb|<b>Figure 4: 1D averaged | + | |valign="top"|[[File:AHL 16h.png|500px|center|thumb|<b>Figure 4: 1D averaged AHL concentration </b> gradient after 11 hours, radial direction]] |
|} | |} | ||
<br> | <br> | ||
Revision as of 22:41, 27 October 2013
Contents |
Circuit containing hydrolases
A seven-species model was implemented to simulate the behaviour of our multicellular sender–receiver system. The model consist of one partial differential equation for AHL and 6 ordinary differential equations with Hill functions that captured the activation of protein synthesis as a function of the concentration of the signalling molecule.
Video: GusA expression levels over 24 hours, concentration in mol/m3. Distance between colonies: 1.5 cm.
For the agar plate and mine cells modules, we use the system of equations and parameters set of the previous simulation.
Mine Cells
The system of differential equations for the states involved in the sender module are given below:
\begin{align} \frac{d[LuxI]}{dt} =\alpha_{LuxI} - d_{LuxI} \cdot [LuxI]\\ \end{align} \begin{align} \frac{d[NagZ]}{dt} =\alpha_{NagZ} - d_{NagZ} \cdot [NagZ]\\ \end{align} \begin{align} \frac{d[AHL]}{dt}= DF \cdot \left(\alpha_{AHL} \cdot LuxI-d_{AHL,i} \cdot [AHL]\right) + C_{agar} \cdot D_{AHL} \nabla^{2} AHL\\ \end{align}
Agar Plate
Two processes take place on the agar: diffusion and decay of AHL. The equation for AHL in agar plate is given below:
\begin{align} \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL-d_{AHL,e} \cdot [AHL]\\ \end{align}
Receiver Cells
Receivers are engineered to respond differently to two AHL concentration levels. Basically, cells should be capable of produce a visible response and in a reasonable amount of time, because the player will need to discriminate between the presence of 0, 1 or 2 adjacent mines. To achieve this goal, we incorporate two enzymatic reporters acting as filters (GusA and AES). The expression of these enzymes is under the control of pLux promoters with different sensitivities, the wild type and a promoter mutant (G1 mutant); The affinity constants for the promoters were obtained from our experimental data. An importan feature of these enzymes is that they can catalyze the hydrolysis of various chromogenic compounds to give rise to a relatively quick coloured response.
The intracellular species of interest in the receiver cells module include: LuxR, AHL, LuxR/AHL complex (denoted as R) and the hydrolases (GusA and AES).
\begin{align} \frac{d[LuxR]}{dt}=\alpha_{[LuxR]} - d_{LuxR} [LuxR] \\ \end{align} \begin{align} \frac{d[R]}{dt}=\rho_{LuxR} \cdot [LuxR ]^2 \cdot [AHL]^2 - d_{R} [R]\\ \end{align} \begin{align} \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL - DF \cdot d_{AHL} \cdot [AHL] \\ \end{align} \begin{align} \frac{d[GusA]}{dt}=\alpha_{GusA} \cdot k_{GusA} + \frac{\alpha_{GusA}(\frac{R}{K_{R_1}})^{n_{1}}}{1+(\frac{R}{K_{R_1}})^{n_{1}}} - d_{GusA}\cdot [GusA]\\ \end{align} \begin{align} \frac{d[AES]}{dt}=\alpha_{AES} \cdot k_{AES} + \frac{\alpha_{AES}(\frac{R}{K_{R_2}})^{n_{2}}}{1+(\frac{R}{K_{R_2}})^{n_{2}}} - d_{AES}\cdot [AES]\\ \end{align}
In addition to new proteins incorporated to the circuit, it is important to emphasize that the grid was changed to a three neighbours setup.
Results
In figure 1 and 3 are shown the results regarding GusA expression levels. From the time course plot (Fig 1.), it can be pointed out that the activation of the gene is consistent with the AHL concentration that is predicted to reach a receiver colony. Additionally, one can say that the incubations of the plates should not be longer than 17 hours, otherwise receiver cells that are more that one cell away from a mine can start synthesizing the enzyme.
Regarding the expression of AES under the control of pLuxR variant (Fig 4.), there is not activation before 15 hours only basal expression. Should be noted that the predicted concentration of enzyme is very low if it is compared to GusA.
 "
"