Team:ETH Zurich/Modeling/Analytical Approximations
From 2013.igem.org
Contents |
Steady State AHL Gradient Approximation
In the reaction-diffusion model we have formalized the gradient establishment and we solved the resulting partial differential equation numerically. Now, the aim is to derive a suitable analytical approximation for a single mine colony.
Kolmogorov-Petrovsky-Piskounov Equation
A reaction–diffusion equation concerning the concentration of a single molecule in one spatial dimension is known as the Kolmogorov-Petrovsky-Piskounov Equation. In our case, the equation for AHL has the following general structure:
\begin{align} \frac{\partial AHL(x,t)}{\partial t} = Diff(AHL(x,t),x) + R(AHL(x,t)) \end{align}
We were interested in the AHL gradient that can be established by a single colony ( located at $x = 0$) on an agar plate, ending up with a less general form of the reaction-diffusion model for AHL:
\begin{align} \frac{\partial AHL}{\partial t} = C_{agar} \cdot D_{AHL} \frac{\partial^2 AHL}{\partial x^2} - d_{AHL,e} \cdot [AHL] \end{align}
As boundary condition, we considered that the concentration at the mine colony stays constant, i.e. we are assuming cells are at the steady state:
\begin{align} [AHL](x=0,t) = [AHL]_{MineCell,ss} = \frac{\alpha_{AHL} \cdot [LuxI]_{ss}} {d_{AHL,i}+\eta \cdot \left(1-\frac{\eta_{ext}}{d_{AHL,e}+\eta_{ext}}\right)} \end{align}
Solution at the steady state
To solve the KPP equation for the steady state, we set to zero the time derivative:
\begin{align} \frac{\partial AHL}{\partial t} = C_{agar} \cdot D_{AHL} \frac{\partial^2 AHL}{\partial x^2} - d_{AHL,e} \cdot [AHL] = 0 \end{align} \begin{align} \frac{\partial^2 AHL}{\partial x^2} = \frac{d_{AHL,e}} {C_{agar} \cdot D_{AHL}} \cdot [AHL] \end{align}
This ordinary differential equation can be solved, integrating wrt. to x twice:
\begin{align}
[AHL] = [AHL]_{MineCell,ss} \cdot e^{-\sqrt{\frac{d_{AHL,e}} {C_{agar} \cdot D_{AHL}}} \cdot x}
\end{align}
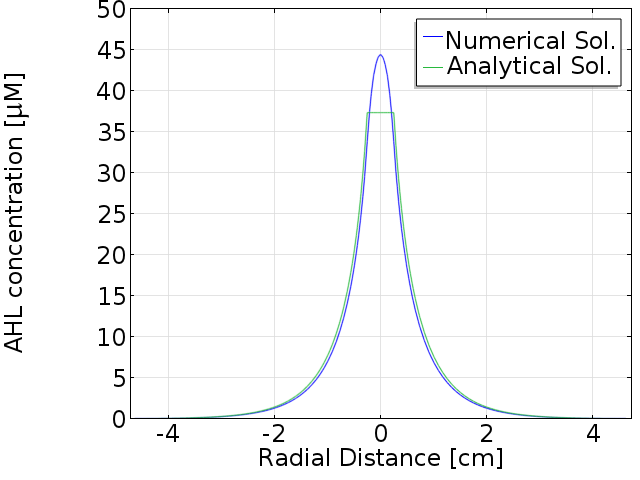
Comparison with numerical solution
Search for an optimal promoter mutant
For the case of 2 neighbouring mines, we need to introduce a second promoter with a different sensitivity towards the complex LuxR/AHL. Experimentally the problem was addressed by constructing a library of promoter mutants. From the model we wanted to evaluate the mutants in order to predict which one could be the best.
In order to reduce the calculations, the current model with 6 species can be simplified to 3 species. Basically, proteins that are constitutively produced and linearly degraded can be assumed to be constants, which is the case for LuxI and LuxR, that are set to the steady state concentration. Regarding the complex LuxR/AHL, we can express production of the output proteins controlled by AHL. Thus, we have senders cells producing AHL and receiver cells processing the signal producing two colour outputs.
Mine Colony
\begin{align} \frac{d[AHL]}{dt} = DF \cdot ( \alpha_{AHL} \cdot [LuxI] - d_{AHL,i} \cdot [AHL] ) + C_{agar} \cdot D_{AHL} \nabla^{2} AHL \end{align}
Agar Plate
\begin{align} \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL-d_{AHL,e} \cdot [AHL] \end{align}
Receiver Colony
\begin{align} \frac{d[AHL]}{dt}= C_{agar} \cdot D_{AHL} \nabla^{2} AHL - DF \cdot (d_{AHL,i} \cdot [AHL]) \\ \end{align}
\begin{align} \frac{d[E_{1}]}{dt}=\alpha_{E_{1}} \cdot k_{basal} \cdot [LuxR]_{ss} + \frac{\alpha_{E_{1}}\left(\frac{AHL}{K_{M_{ wt}}}\right)^{2}}{1+\left(\frac{AHL}{K_{M_{ wt}}}\right)^{2}} - d_{E_{1}}\cdot [E_{1}]\\ \end{align}
\begin{align} \frac{d[E_{2}]}{dt}=\alpha_{E_{2}} \cdot k_{basal} \cdot [LuxR]_{ss} + \frac{\alpha_{E_{2}}\left(\frac{AHL}{K_{M_{ mut}}}\right)^{2}}{1+\left(\frac{AHL}{K_{M_{ mut}}}\right)^{2}} - d_{E_{2}}\cdot [E_{2}]\\ \end{align}
$E_{1}$ an $E_{2}$ is a abstract way to indicate the output system for the case of 1 or 2 neighbouring mines. The output system can consist either of fluorescent proteins, GFP and RFP, or hydrolases, GusA and AES.
The second protein, $E_{2}$, is responsible to give a colour output that indicate the presence of two mines. Its synthesis should start with some delayed after the first protein, given that the promoter is less sensitive to AHL. However, this protein is also expressed by colonies surrounded by a single mine colony. Our goal is to ensure that the ratio of expression levels for 2 to 1 neighbouring mines are large enough. Taking the limit for this ratio we got:
\begin{align} \lim_{K_{M_{ mut}} \to \infty} \frac{\frac{\left(\frac{AHL_{2}}{K_{M_{ mut}}}\right)^{2}}{1+\left(\frac{AHL_{2}}{K_{M_{ mut}}}\right)^{2}}}{\frac{\left(\frac{AHL_{1}}{K_{M_{ mut}}}\right)^{2}}{1+\left(\frac{AHL_{1}}{K_{M_{ mut}}}\right)^{2}}} = \lim_{K_{M_{ mut}} \to \infty} \left(\frac{AHL_{2}}{AHL_{1}} \right)^2 \frac{K_{M_{ mut}}^2 + AHL_{1}^2}{K_{M_{ mut}}^2 + AHL_{2}^2} = \left(\frac{AHL_{2}}{AHL_{1}} \right)^2 \end{align}
\begin{align} \lim_{K_{M_{ mut}} \to \infty} \frac {\left( \frac{AHL_{2}}{K_{M_{ mut}}}\right)^{2}} {1+\left(\frac{AHL_{2}}{K_{M_{ mut}}}\right)^{2}} \Bigg / \frac{\left(\frac{AHL_{1}}{K_{M_{ mut}}}\right)^{2}}{1+\left(\frac{AHL_{1}}{K_{M_{ mut}}}\right)^{2}} = \lim_{K_{M_{ mut}} \to \infty} \left(\frac{AHL_{2}}{AHL_{1}} \right)^2 \frac{K_{M_{ mut}}^2 + AHL_{1}^2}{K_{M_{ mut}}^2 + AHL_{2}^2} = \left(\frac{AHL_{2}}{AHL_{1}} \right)^2 \end{align}
 "
"