Team:SJTU-BioX-Shanghai/Project/Regulator/Integrating
From 2013.igem.org
(Difference between revisions)
| Line 27: | Line 27: | ||
__NOTOC__ | __NOTOC__ | ||
<!----------------------------------------------------从这里开始写wiki---------------------------------> | <!----------------------------------------------------从这里开始写wiki---------------------------------> | ||
| + | =IDEA!= | ||
| + | {{Template:13SJTU_project_summary_head}} | ||
| + | Place sgRNAs under control of different light sensors. '''One light one sgRNA'''. | ||
| + | <br><br> | ||
| + | Our system operates like this: | ||
| + | <br> | ||
| + | 1. light sensors regulates sgRNAs; | ||
| + | <br> | ||
| + | 2. then sgRNAs regulate their corresponding genes, which is the final targets in the pathway to be optimized. | ||
| + | <br><br> | ||
| + | Therefore, we are able to: | ||
| + | * Regulate '''Genomic''' Genes. | ||
| + | In the past, researchers seldom accomplishes the regulation of genomic genes since it is so difficult to change endogenous promoters to be regulated by outer signals. | ||
| + | * Simultaneously controlling '''several''' genes. | ||
| + | Each gene is regulated by a different light, respectively. | ||
| + | * Offer a '''easy-to-use''' platform. | ||
| + | Users of our Metabolic Gear Box can optimize their own desired pathway after making some minor changes to a vector of sgRNAs. | ||
| + | {{Template:12SJTU_part_summary_foot}} | ||
| + | <br> | ||
| - | = | + | =Testing CRISPRi= |
<br> | <br> | ||
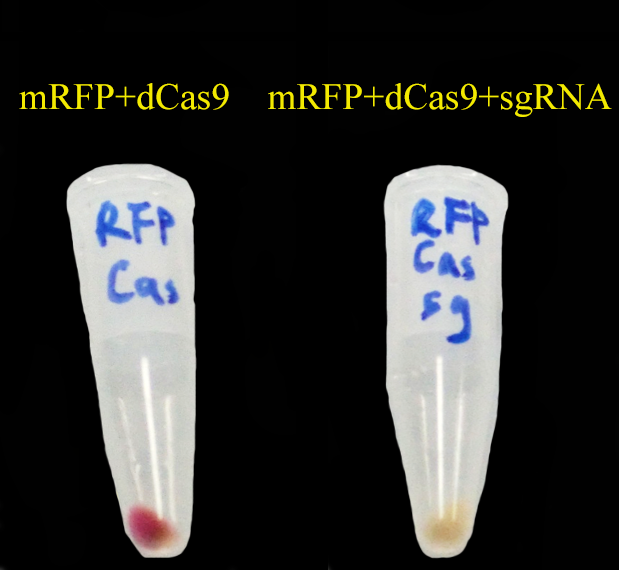
| - | + | To test CRISPRi, we have constructed a '''constitutive''' dCas9 on pRSFDuet, a '''constitutive sgRNA targeting mRFP (gR4mRFP)''' on pCDFDuet and a constitutive mRFP on pETDuet. | |
| - | [[File: | + | Then we set up a strictly controlled experiment as follows: |
| + | * Case: constitutive dCas9 on pRSFDuet + '''a constitutive gR4mRFP on pCDFDuet''' + constitutive mRFP on pETDuet | ||
| + | * Controll: constitutive dCas9 on pRSFDuet + ''' an empty pCDFDuet ''' + constitutive mRFP on pETDuet | ||
| + | [[File:CRISPRi_test.png]] | ||
<br> | <br> | ||
| - | + | As expected, '''mRFP repressed by CRISPRi (dCas9 and gR4mRFP) is not as red as the control.''' | |
| - | + | <br><br> | |
| - | + | The sequence of gR4mRPF is given in our part BBa_K1026003 . This sequence comes from the following literature: QI, LEI S., LARSON, MATTHEW H., GILBERT, LUKE A., DOUDNA, JENNIFER A., WEISSMAN, JONATHAN S., ARKIN, ADAM P. & LIM, WENDELL A. 2013. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell, 152, 1173-1183. | |
| - | + | <br><br> | |
| + | And <font size=4>'''CAUTION'''</font> that: | ||
| + | * There shall '''not''' be extra nucleotides between promoter and sgRNA itself. | ||
| + | gRNA shall be exactly transcribed out. Any extra nucleotides would potentially jeopardize the normal function of the gRNA. Therefore, '''BioBrick scars are not allowed here'''. Instead of cut-and-ligation, inverse PCR or In-Fusion is preferred for the assembly task. | ||
| + | Our part '''BBa_K1026003''' can be an example of no-scar assembly. | ||
| + | * A reliable terminator is applied here to '''ensure the termination of sgRNA'''. | ||
<br> | <br> | ||
| - | + | <br><br> | |
| - | <br><br> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | = | + | =Testing the '''Interface''' of CRISPRi and Light Sensors= |
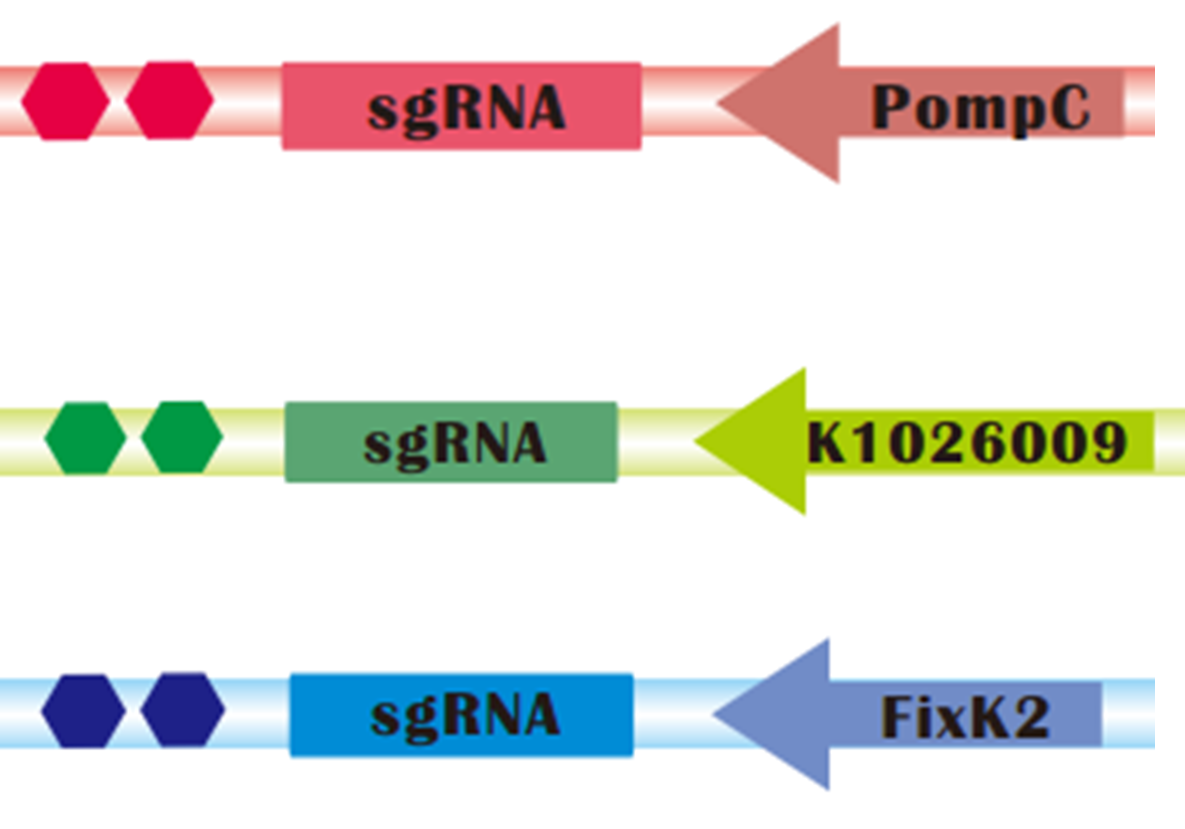
| + | [[File:SgRNA_operons.png|thumb|300px|right|sgRNAs regulated by light sensors. Up: Red light controlled sgRNA; Medium: Green light controlled sgRNA; Down: Blue light controlled sgRNA]] | ||
<br> | <br> | ||
| - | + | To assure that CRISPRi can be successfully combined with Light Sensors, we constructed '''sgRNAs targeting mRFP that are regulated by <font color=red>red</font>, <font color=green>green</font> and <font color=blue>blue light sensors</font>, respectively''': | |
| + | * POmpC is a promoter negatively regulated by Red Light. | ||
| + | * K1026009, one of our parts, is a artificial promoter negatively regulated by green light. | ||
| + | * FixK2 is a promoter negatively regulated by Blue Light. | ||
| + | <br> | ||
| + | |||
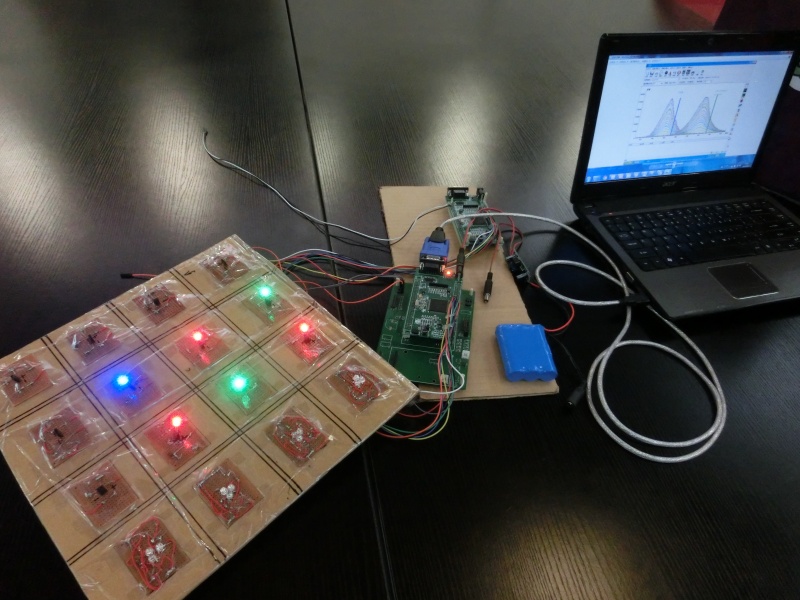
| + | And next, it is show time for our Metabolic Gear Box! | ||
| + | [[File:Metabolic_Gear_Box_with_Culture.JPG|thumb||700px|A Metabolic Gear Box in work! With bacteria culture! And in a thermostat incubator!]] | ||
| + | [[File:Metabolic_Gear_Box_Controlling_System.JPG|thumb|700px|A Metabolic Gear Box Receiving Feed-forward Commands. Researchers will now send commands of desired expression level to the computer. Then these commands will be transferred to the Box]] | ||
| + | <br><br><br><br><br><br> | ||
| + | Since it is difficult for RFP reporter to accurately produce qualitative results, we continue to the next phase of our project -- luciferase reporter assay. | ||
<br><br> | <br><br> | ||
| - | |||
<br><br> | <br><br> | ||
| - | + | ||
| - | + | =Gathering '''Quantitative''' Data -- sgRNA targeting '''luciferase'''= | |
| + | <br> | ||
| + | In order to know '''whether our system provides a wide enough range of continuous adjustment''', we constructed sgRNAs targeting luciferase (gR4luciferase) gene. And again, these gR4luciferase are under controll of red, green and blue light, respectively. | ||
<br><br> | <br><br> | ||
| - | <font size=4>sgRNA</font size=4> | + | <font size=4>Designing a sgRNA targeting luciferase</font size=4> |
| - | + | ||
<br> | <br> | ||
| - | + | Since we do not know the sequence of a working sgRNA that targets luciferase, so we designed two gR4mRFP that complements the coding sequence of luciferase gene ourselves according to those design criteria: one is '''closer to the start codon (the proximal one)''', whereas the other is relatively further (the '''distal one'''). | |
| - | + | However it is necessary for us to test these sgRNA. So we again inserted these two sgRNAs into a constitutive operon. After conducting a luciferase reporter assay with a Beyotime kit, it comes out that the repression effect of these two sgRNAs are 80% and 60%, respectively. | |
| - | + | Therefore, we choose the proximal one for later use. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br><br> | <br><br> | ||
| - | + | <font size=4>Determining the '''Working Curve''' of our Metabolic Gear Box</font size=4> | |
| - | < | + | |
| - | + | ||
| - | + | ||
<br> | <br> | ||
| - | + | Again, it is time for our Metabolic Gear Box. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<!----------------------------------------------------到这里结束---------------------------------------> | <!----------------------------------------------------到这里结束---------------------------------------> | ||
</td> | </td> | ||
Revision as of 00:29, 28 September 2013
 "
"