Team:Heidelberg/Delftibactin/Delftibactin
From 2013.igem.org
| Line 58: | Line 58: | ||
<p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | ||
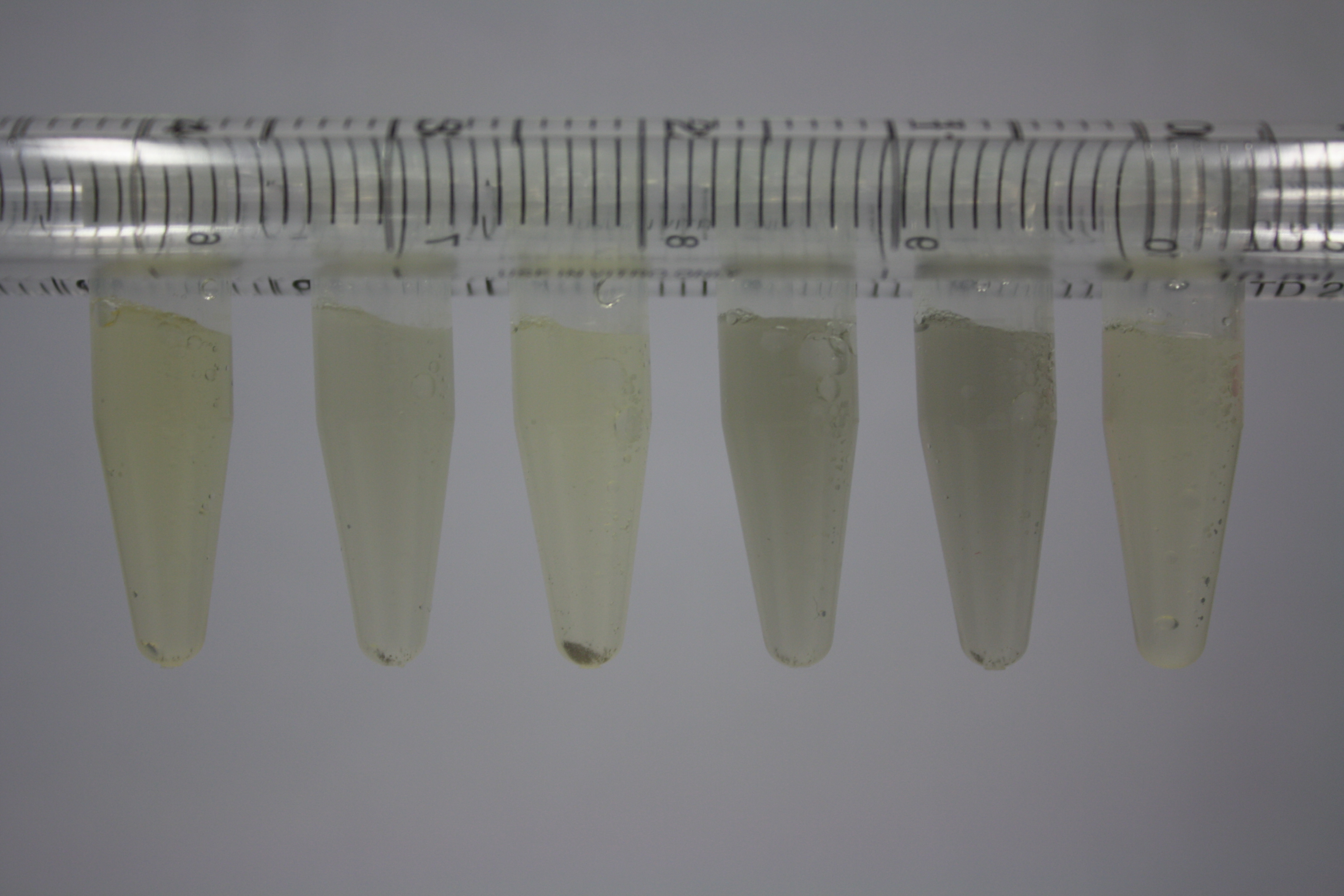
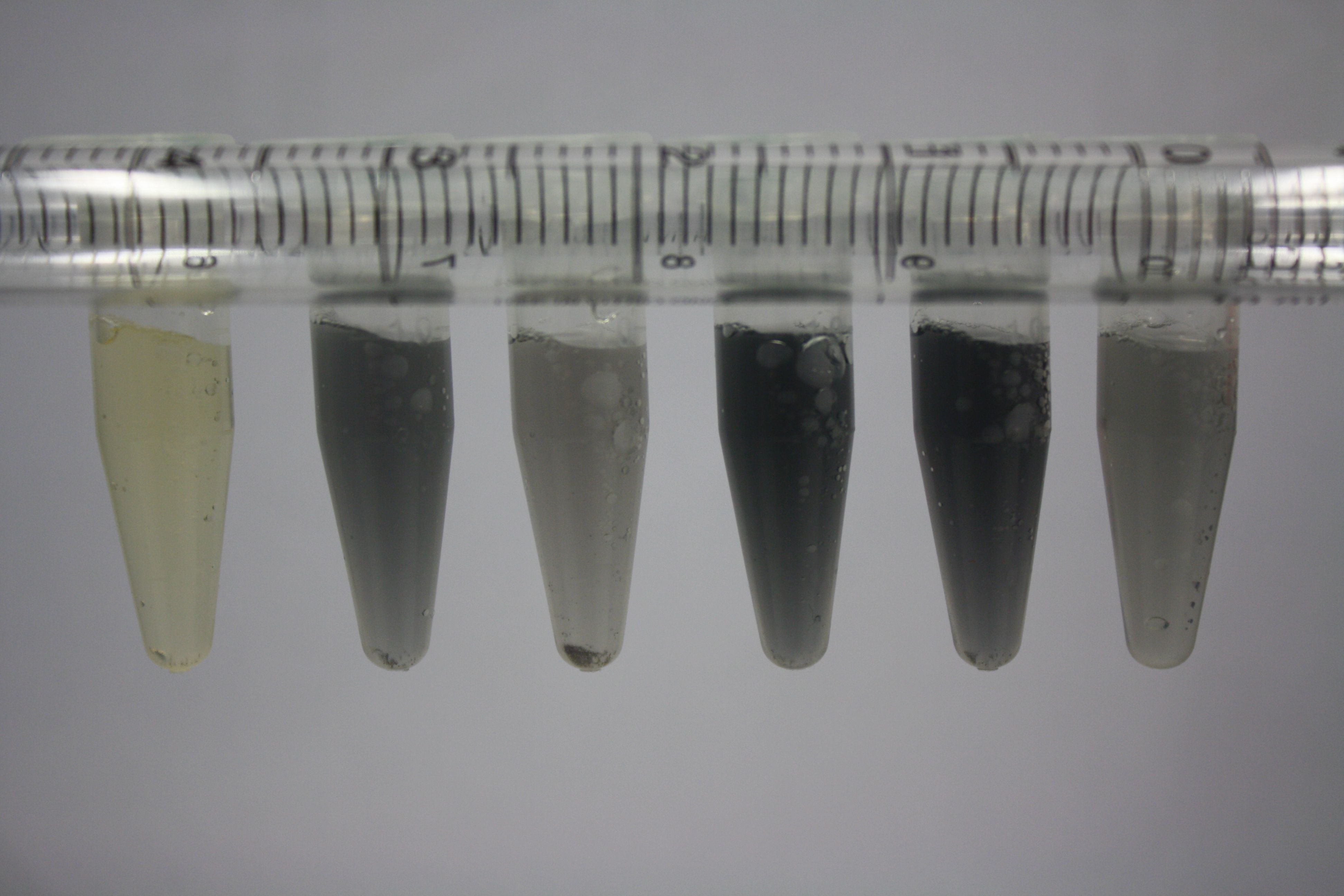
| - | + | We obtained D. acidovorans DSM-39 from the ZSMZ and successfully reproduced the paper of Johnsson et al. D. acidovorans is capable to precipitate solid gold from gold chloride solution as purple-black nanoparticles. Already at low concentrations of gold chloride, gold nonaparticles are precipitated increasing with concentration of gold chloride in solution. In our experiments, precipitation on agar plates worked even better than described in the paper. | |
</p> | </p> | ||
| Line 68: | Line 68: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 3</h1> | <h1>Week 3</h1> | ||
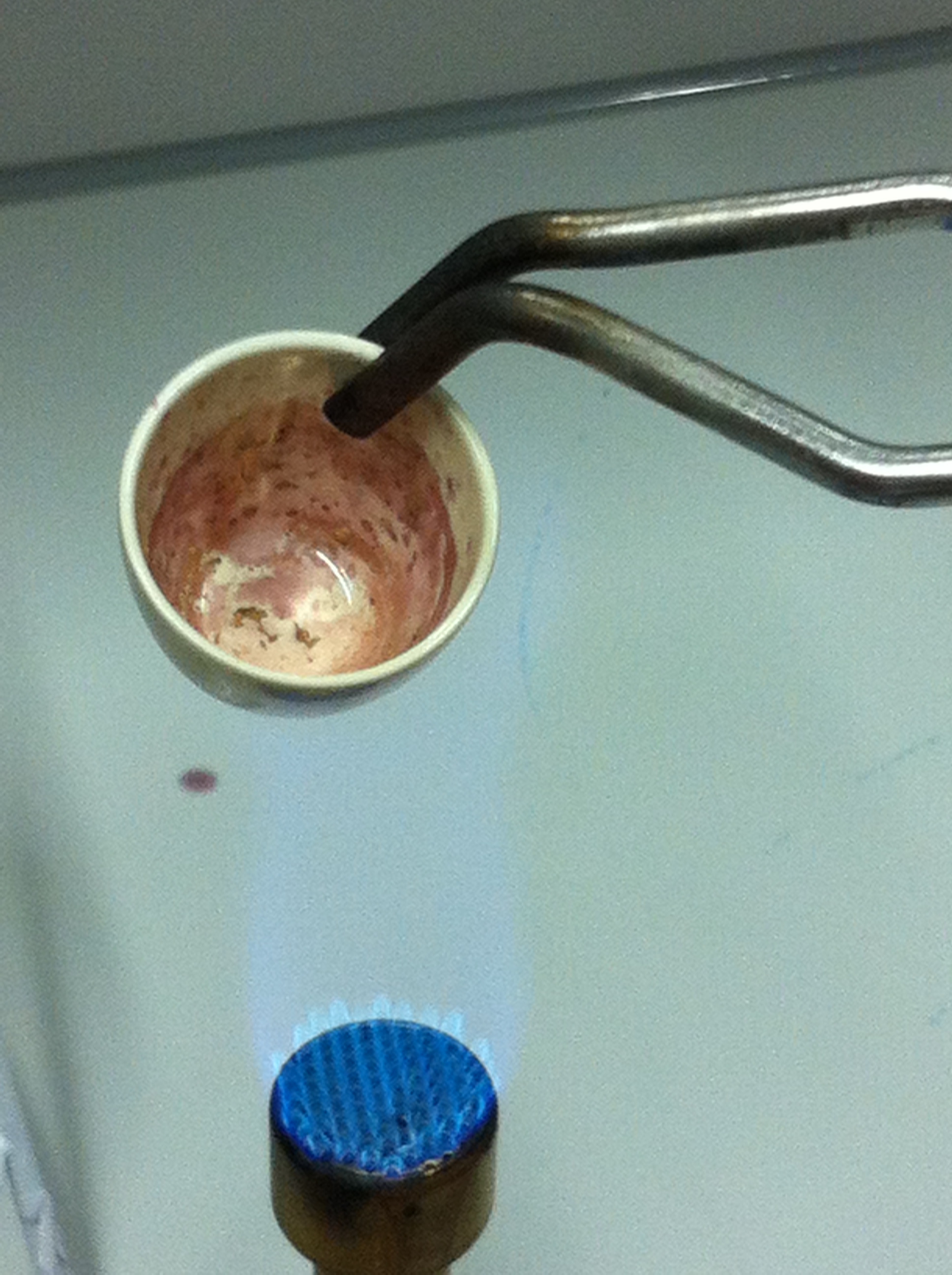
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">We were able to dissolve gold-containing parts of an old CPU and established a protocol for recovery of gold as soluble gold salts from electronic waste. |
</p> | </p> | ||
</div> | </div> | ||
| Line 78: | Line 78: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 15</h1> | <h1>Week 15</h1> | ||
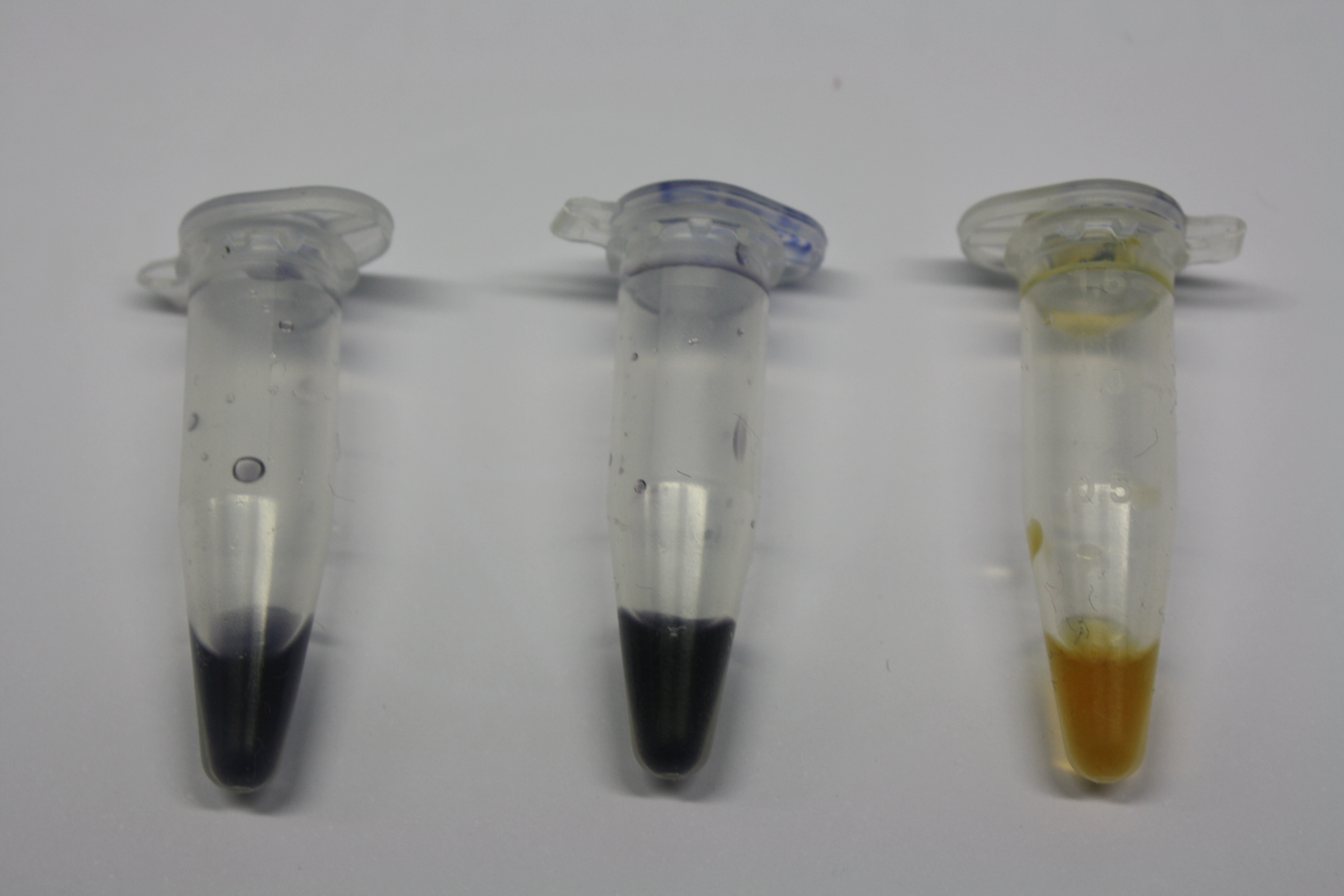
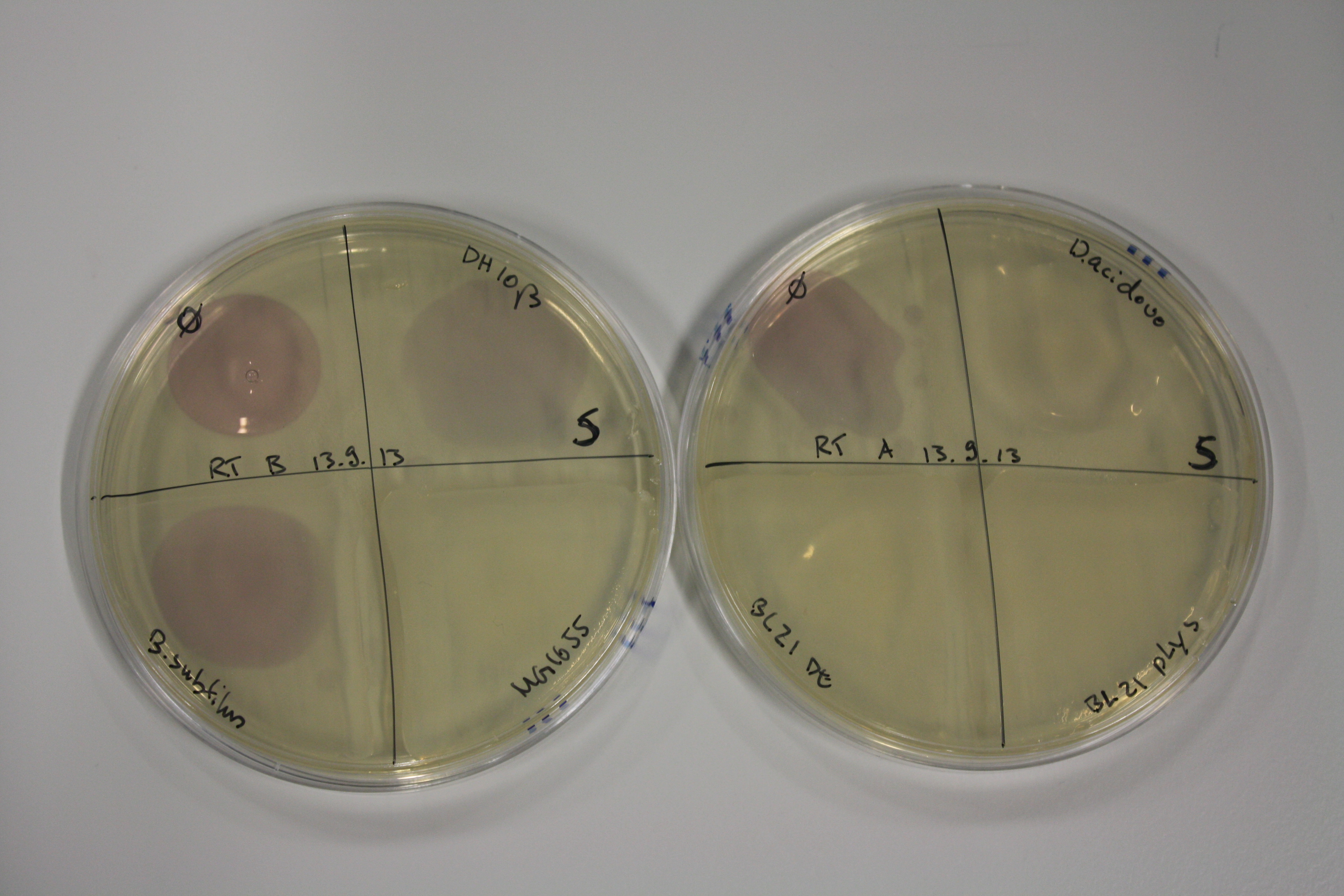
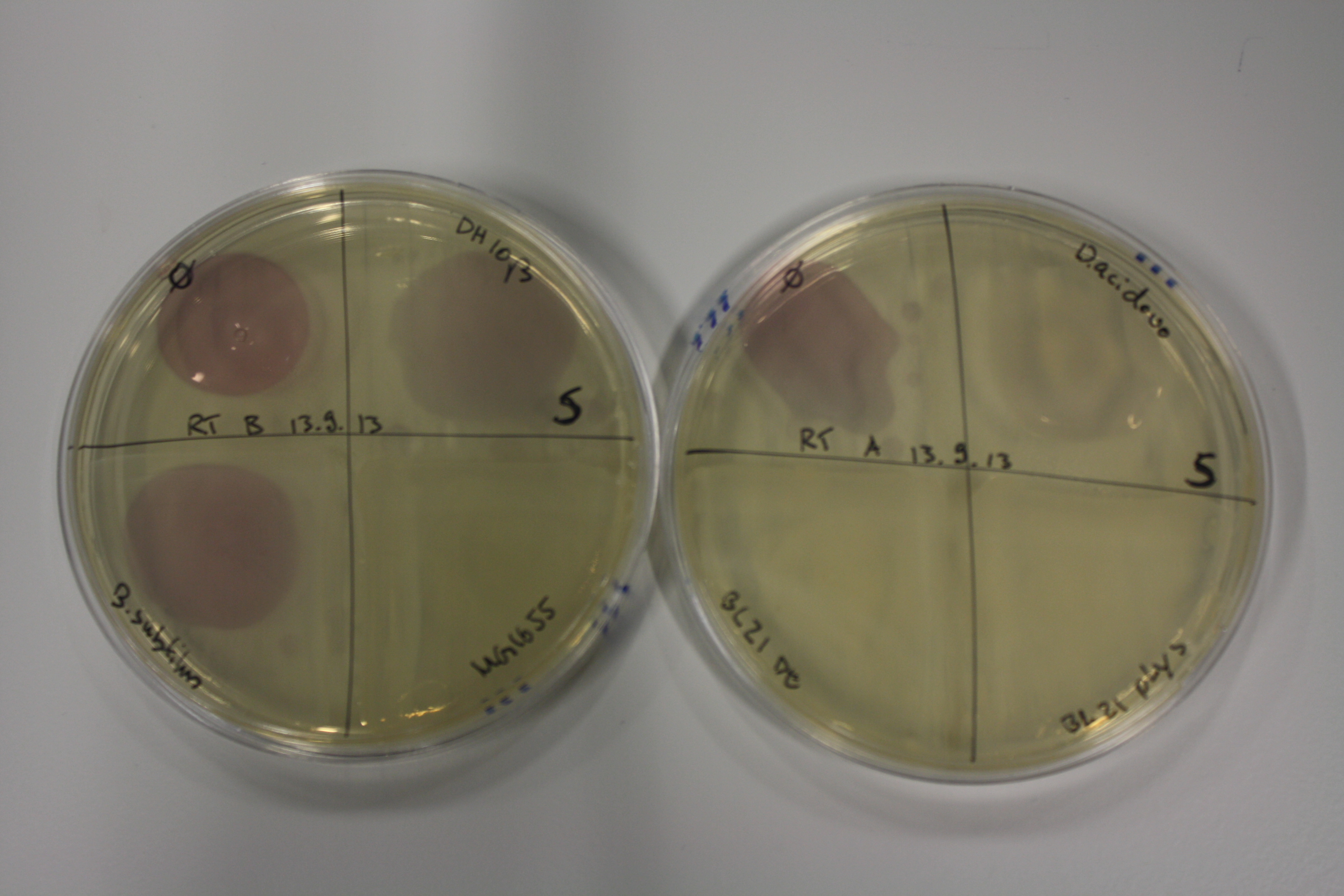
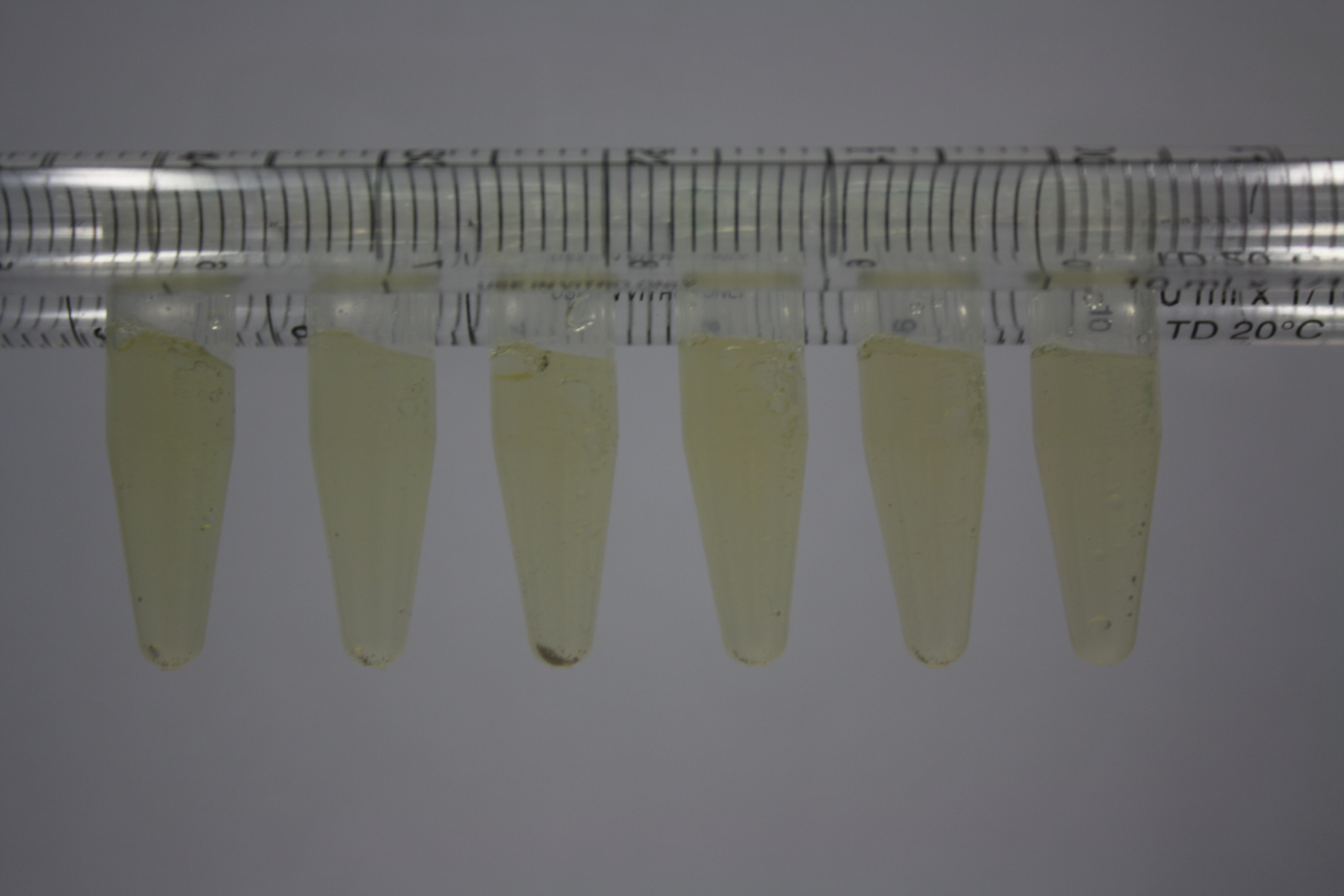
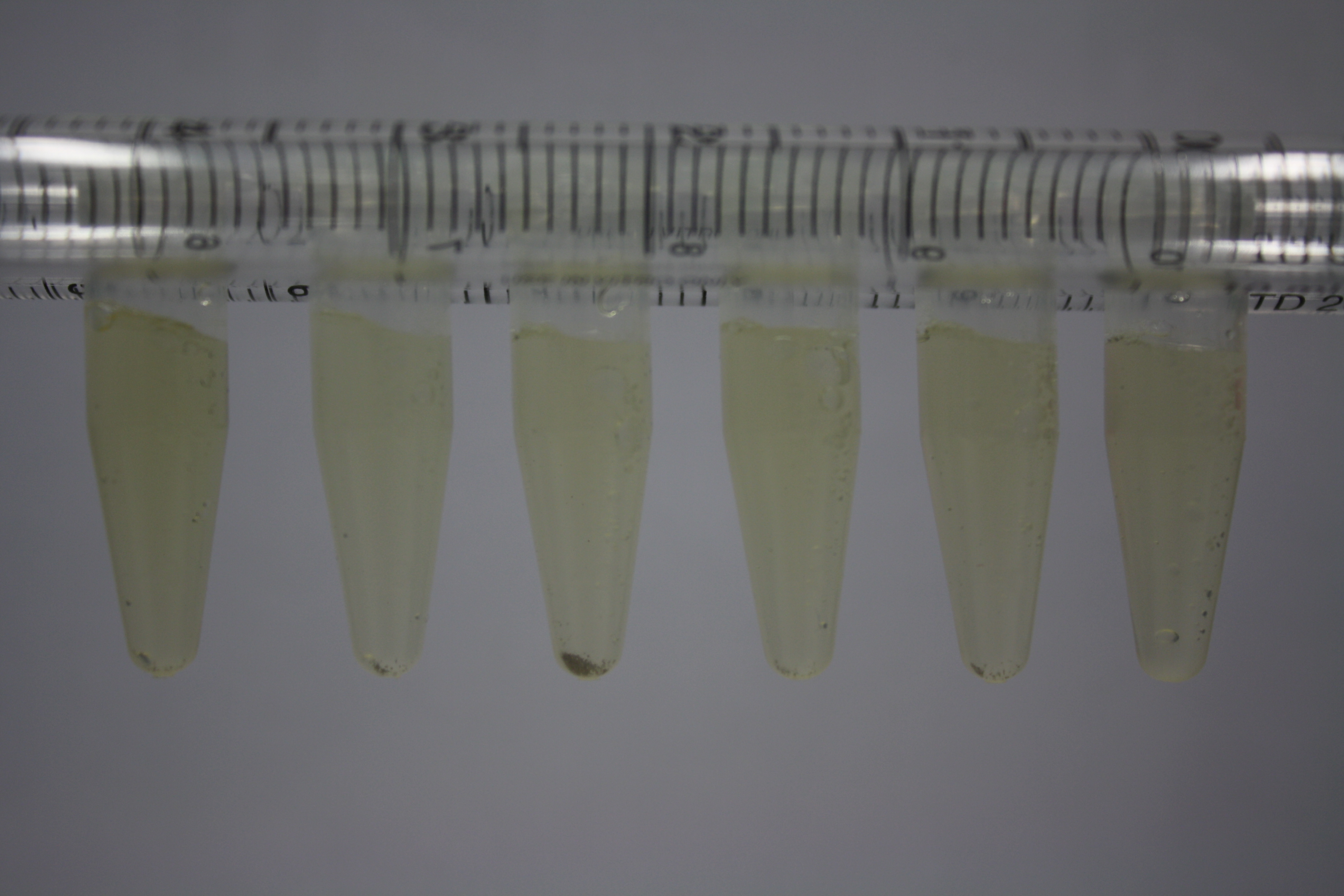
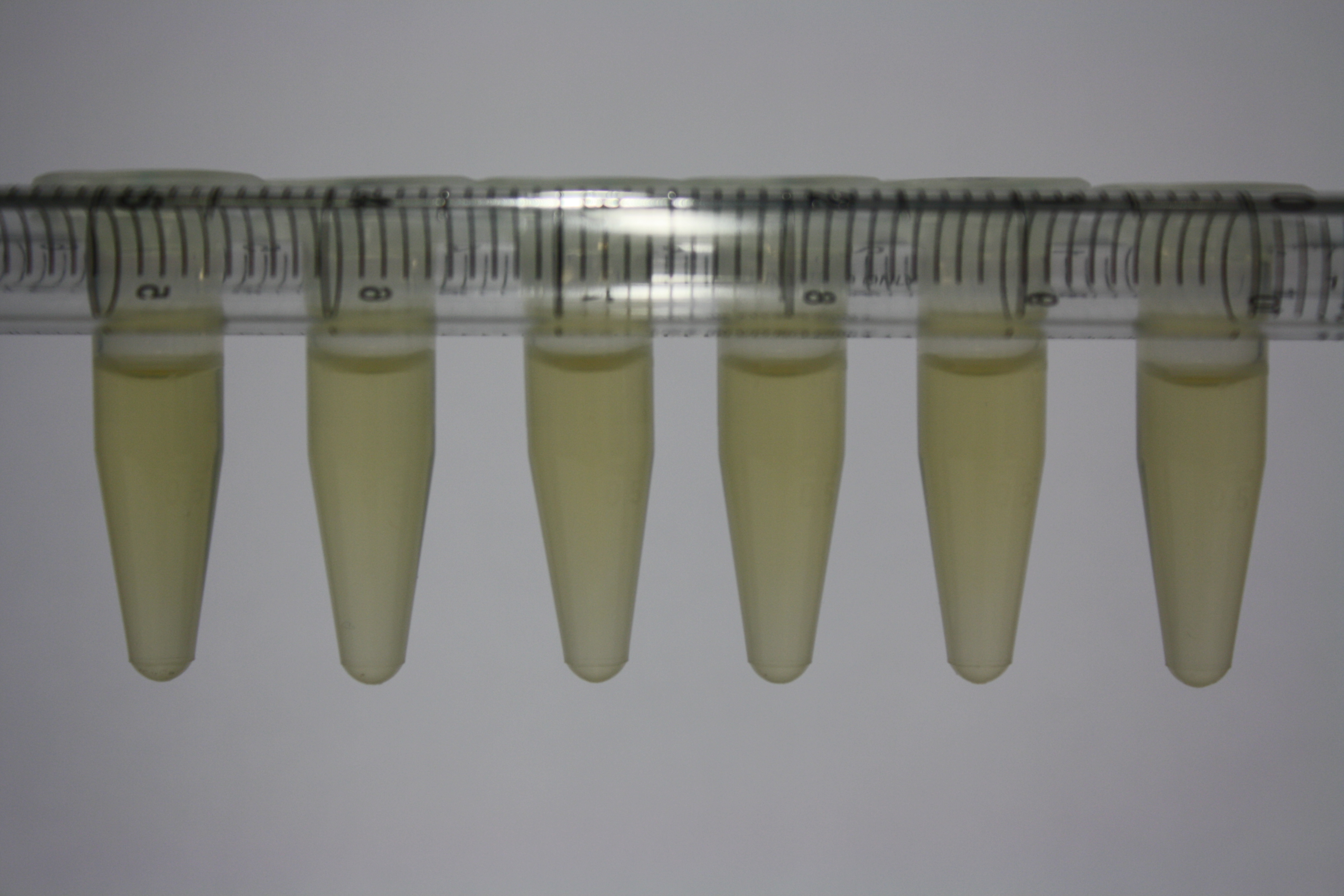
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">Using supernatants from the new Delftia acidovorans strain SPH-1, we showed precipitation of gold chloride solution to gold nanoparticles. Furthermore, we melted the purple-black nanoparticles to shiny solid gold. </p> |
</div> | </div> | ||
</div> | </div> | ||
| Line 87: | Line 87: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 19</h1> | <h1>Week 19</h1> | ||
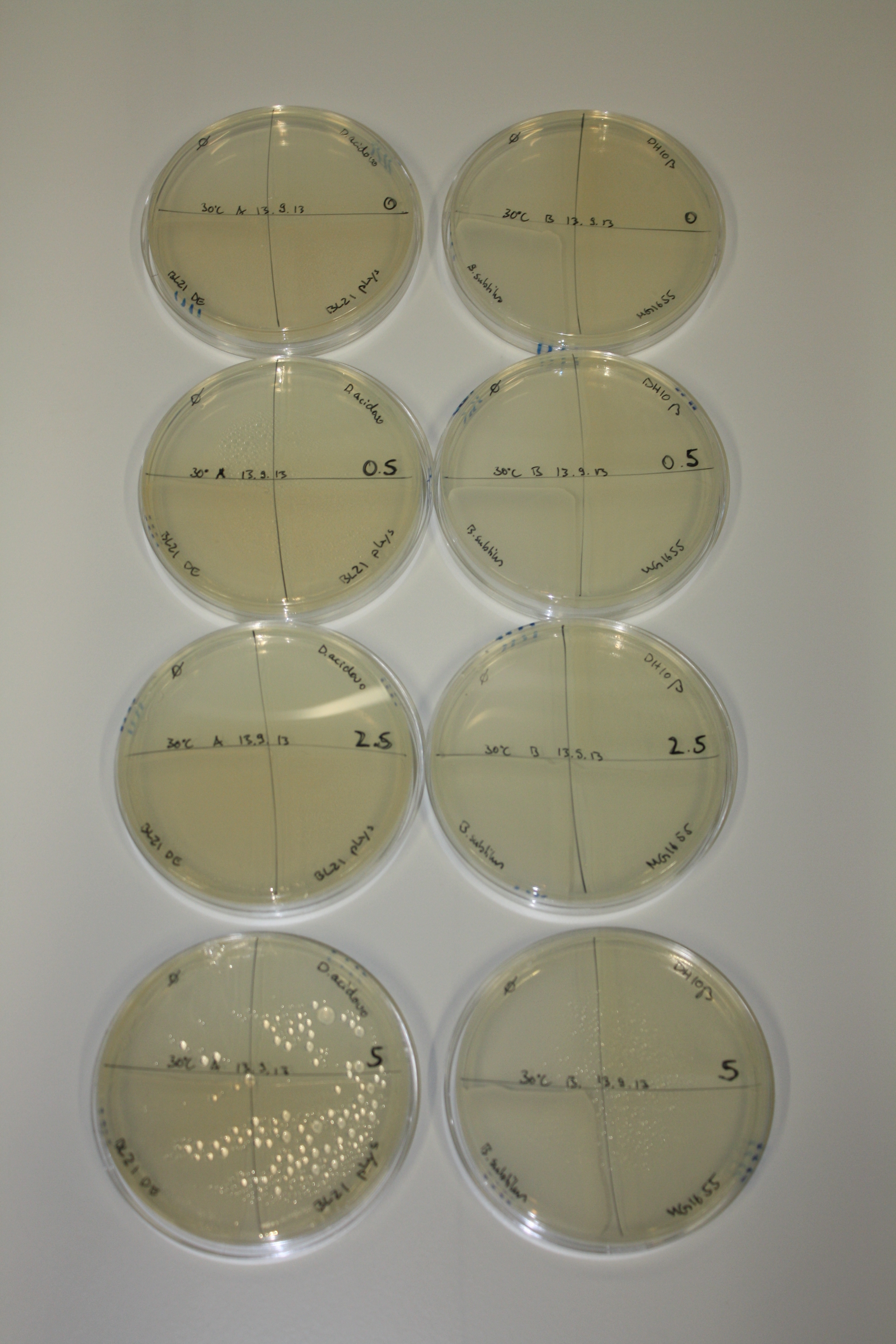
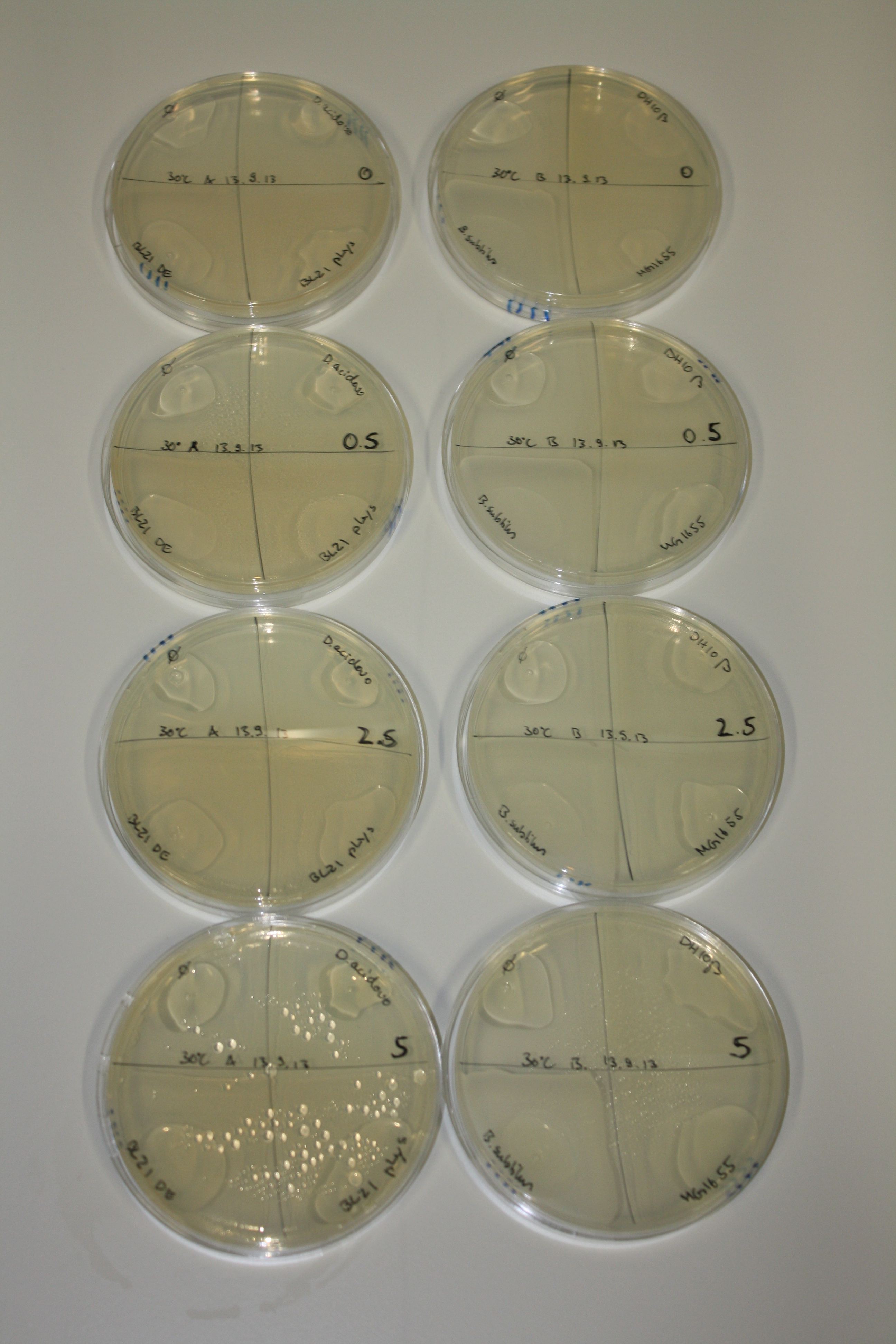
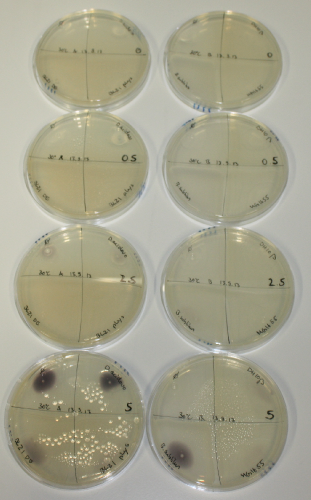
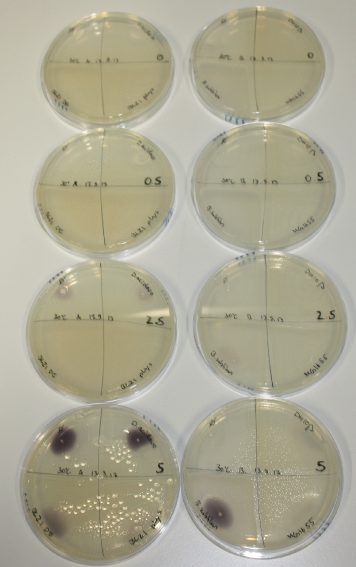
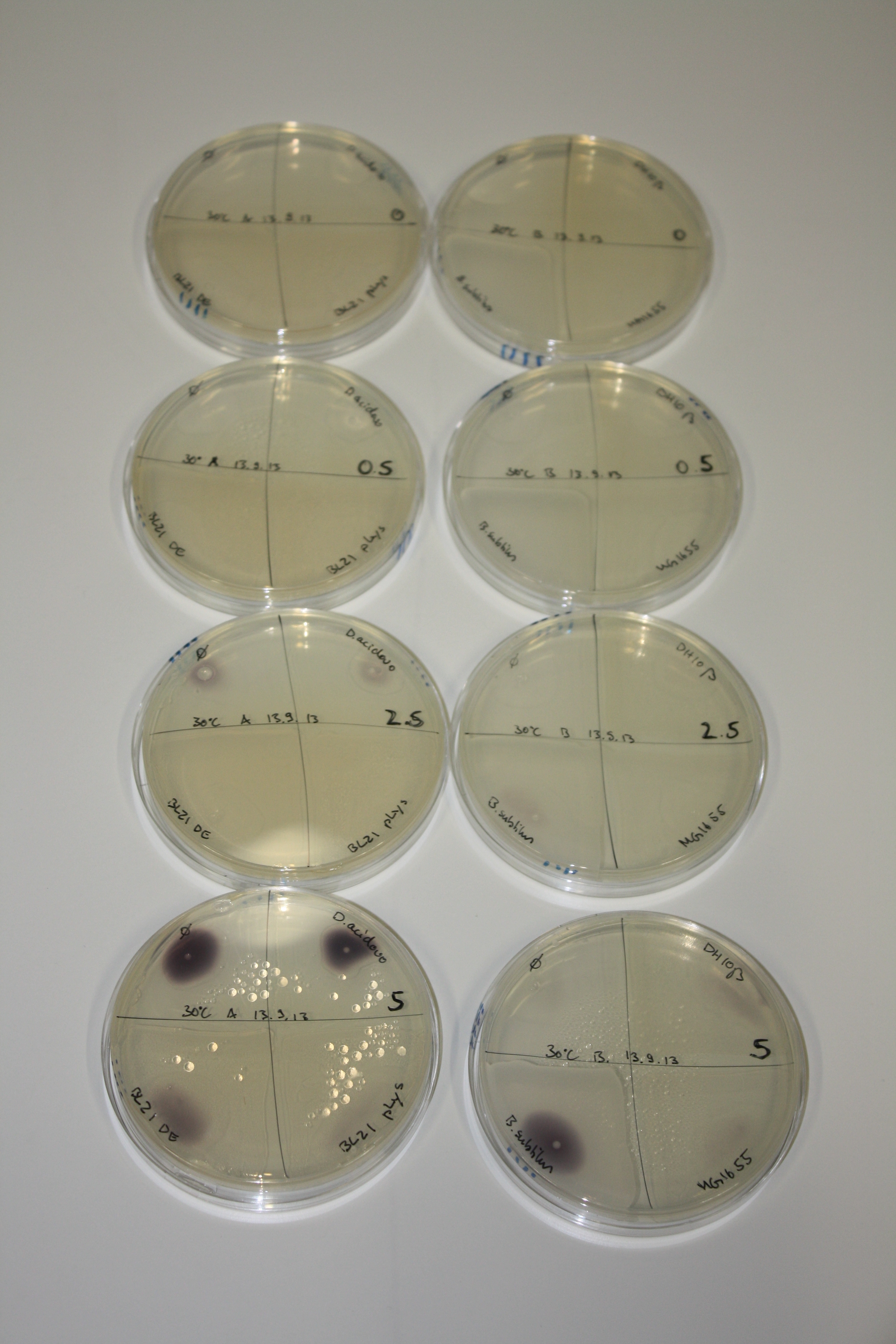
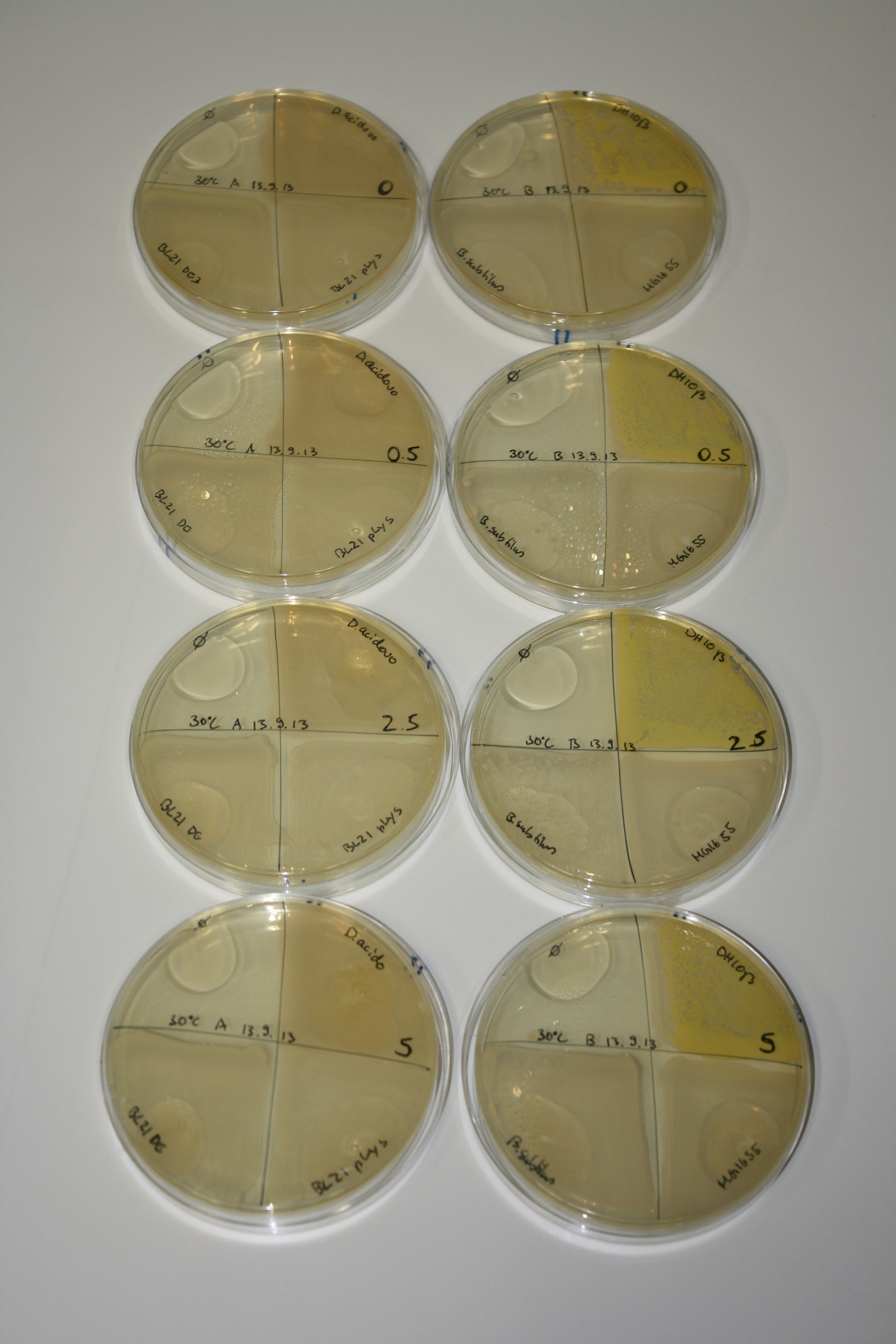
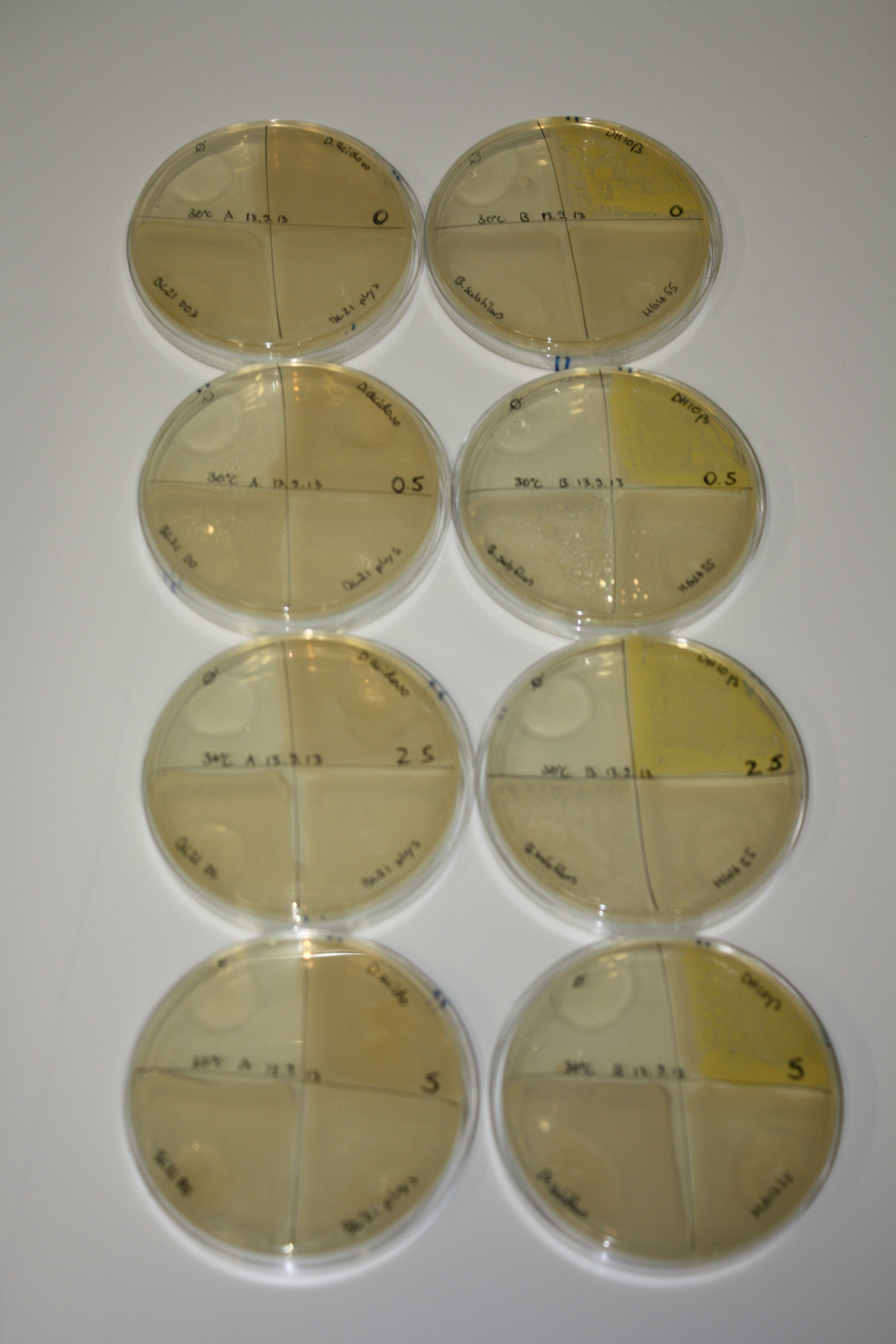
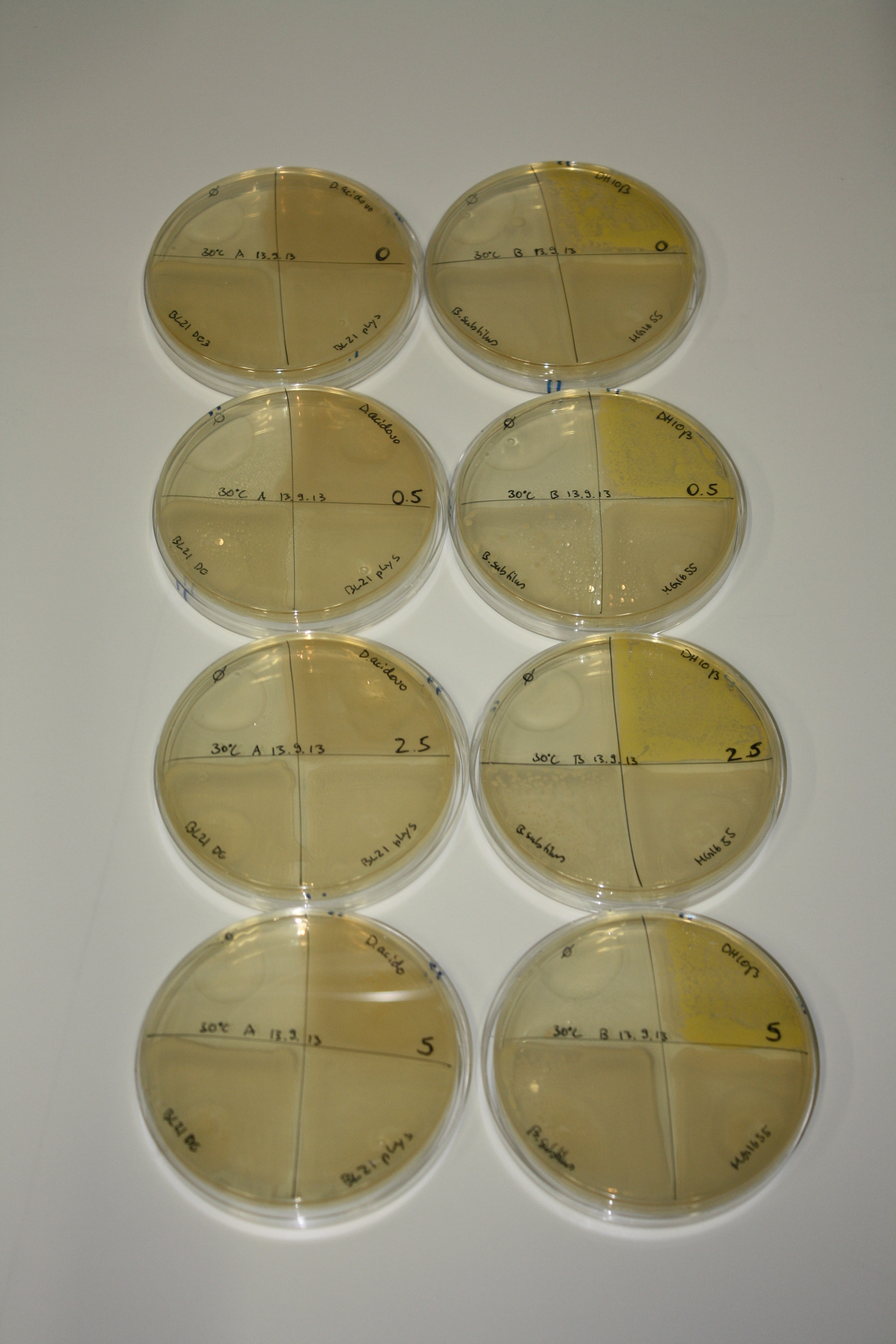
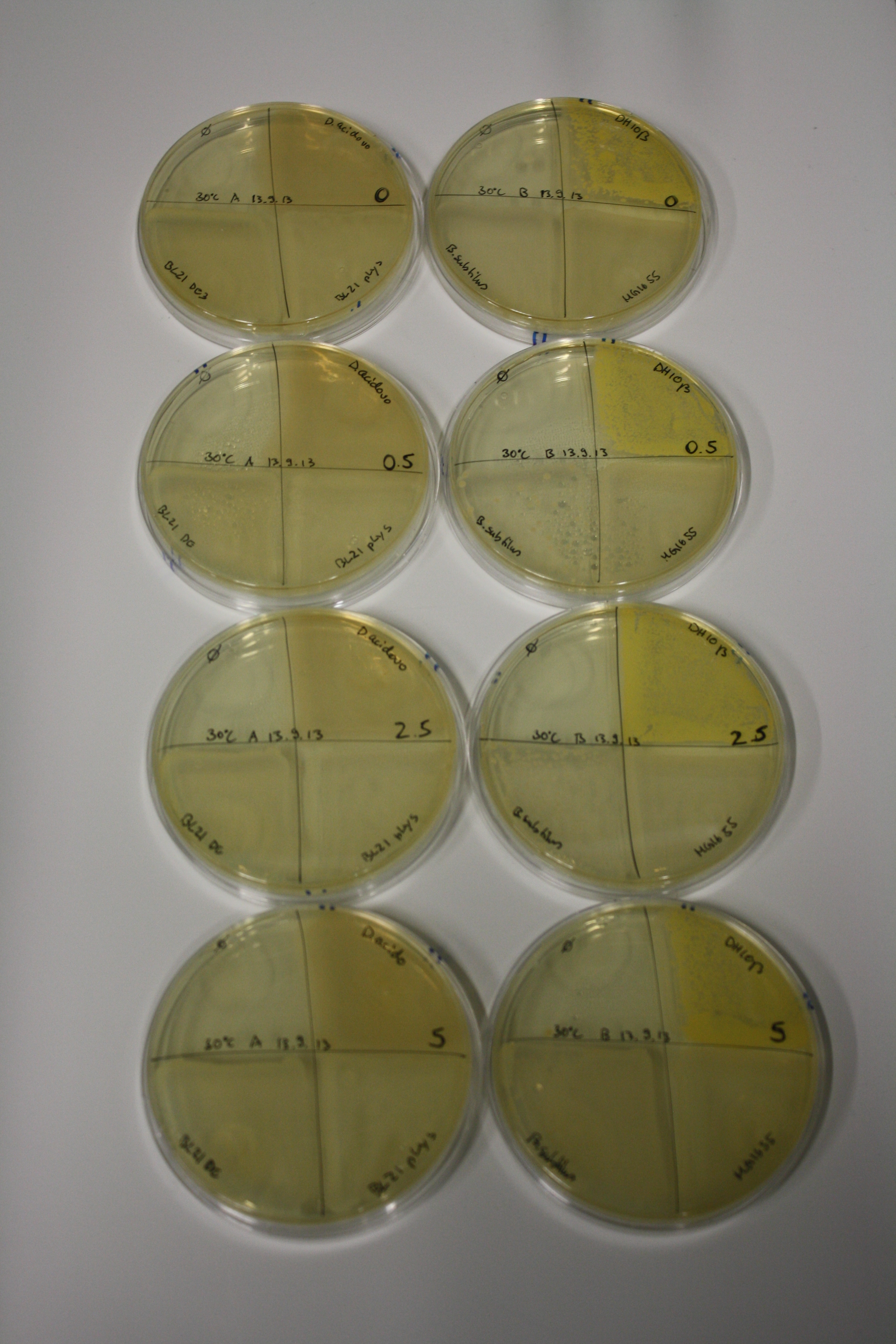
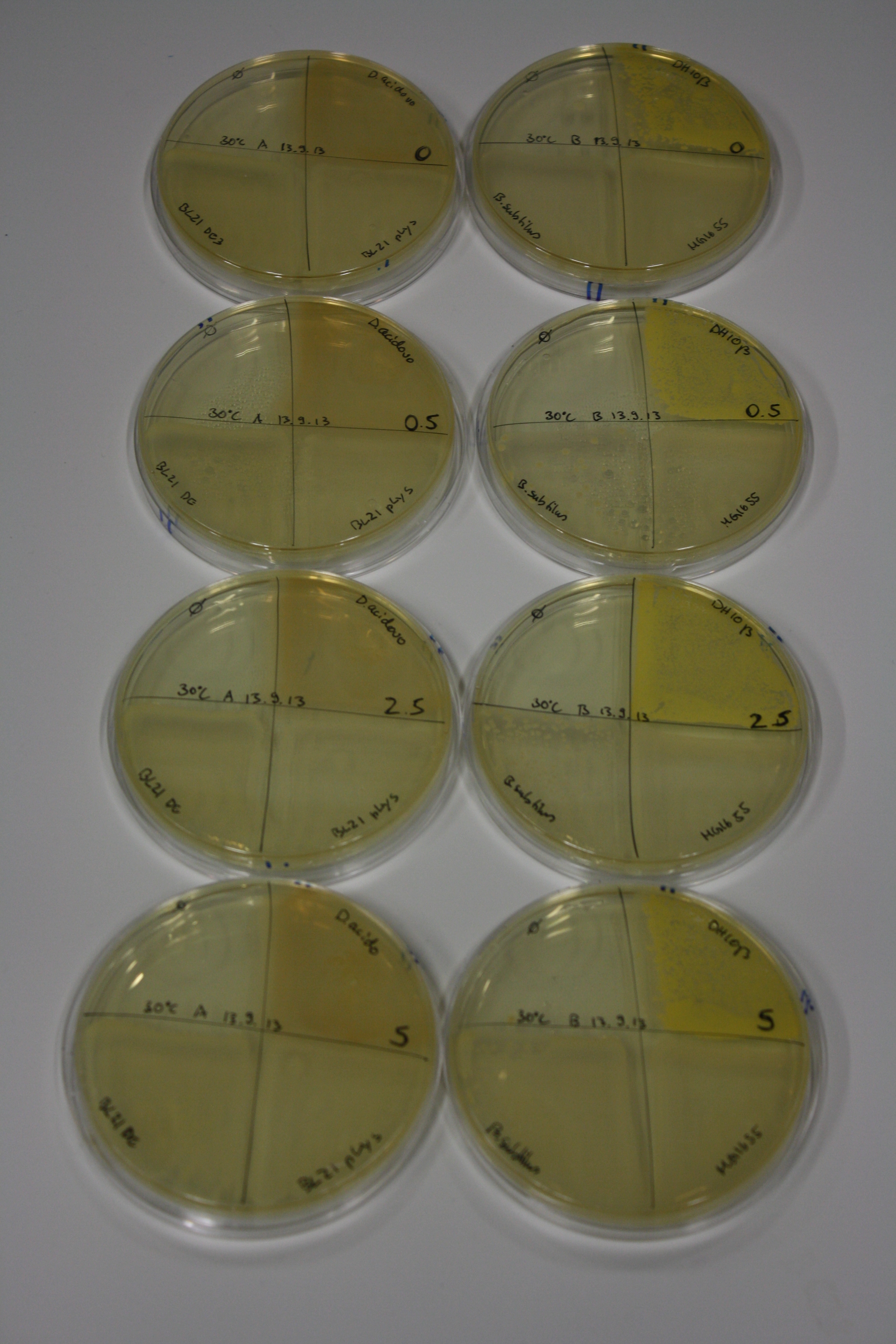
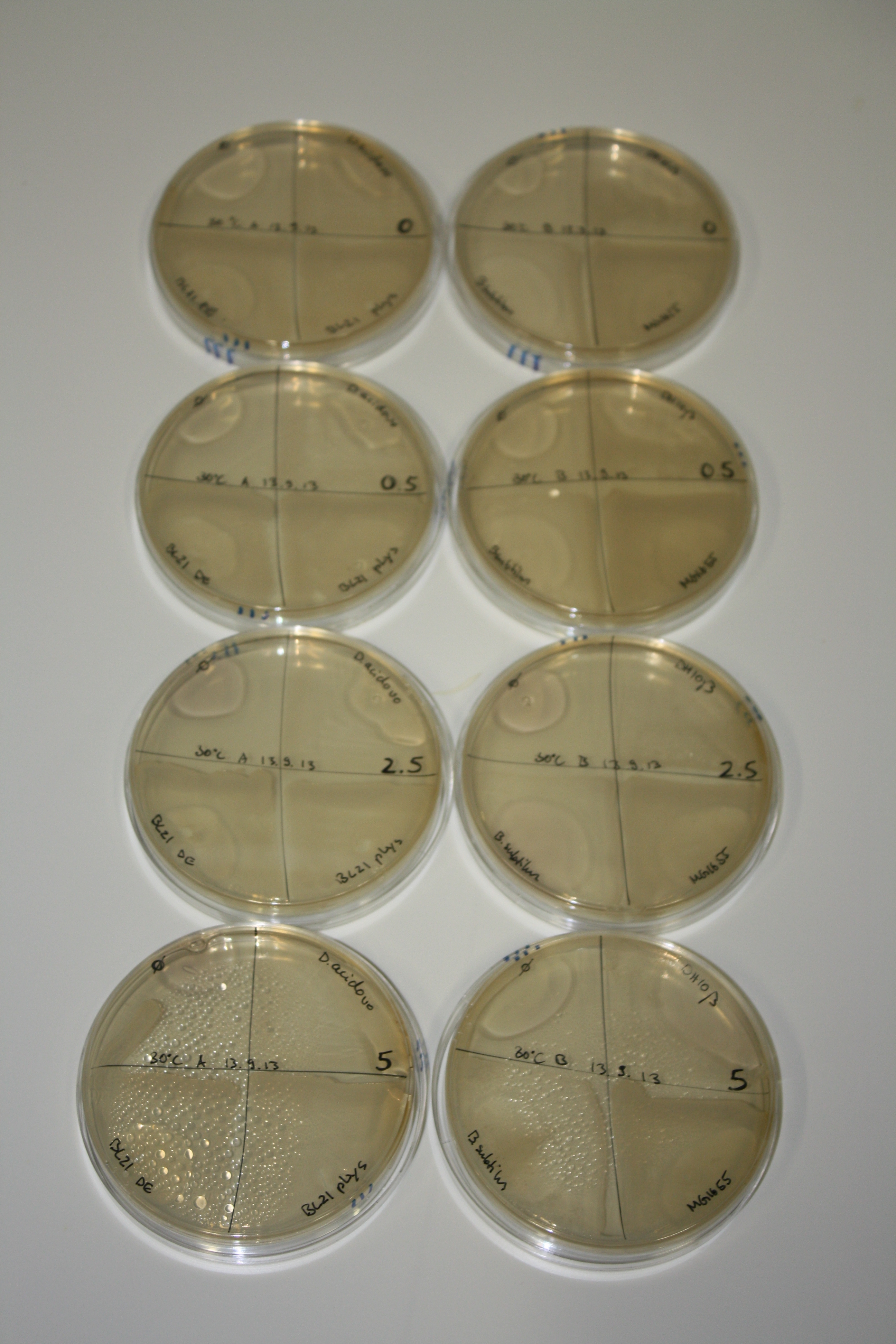
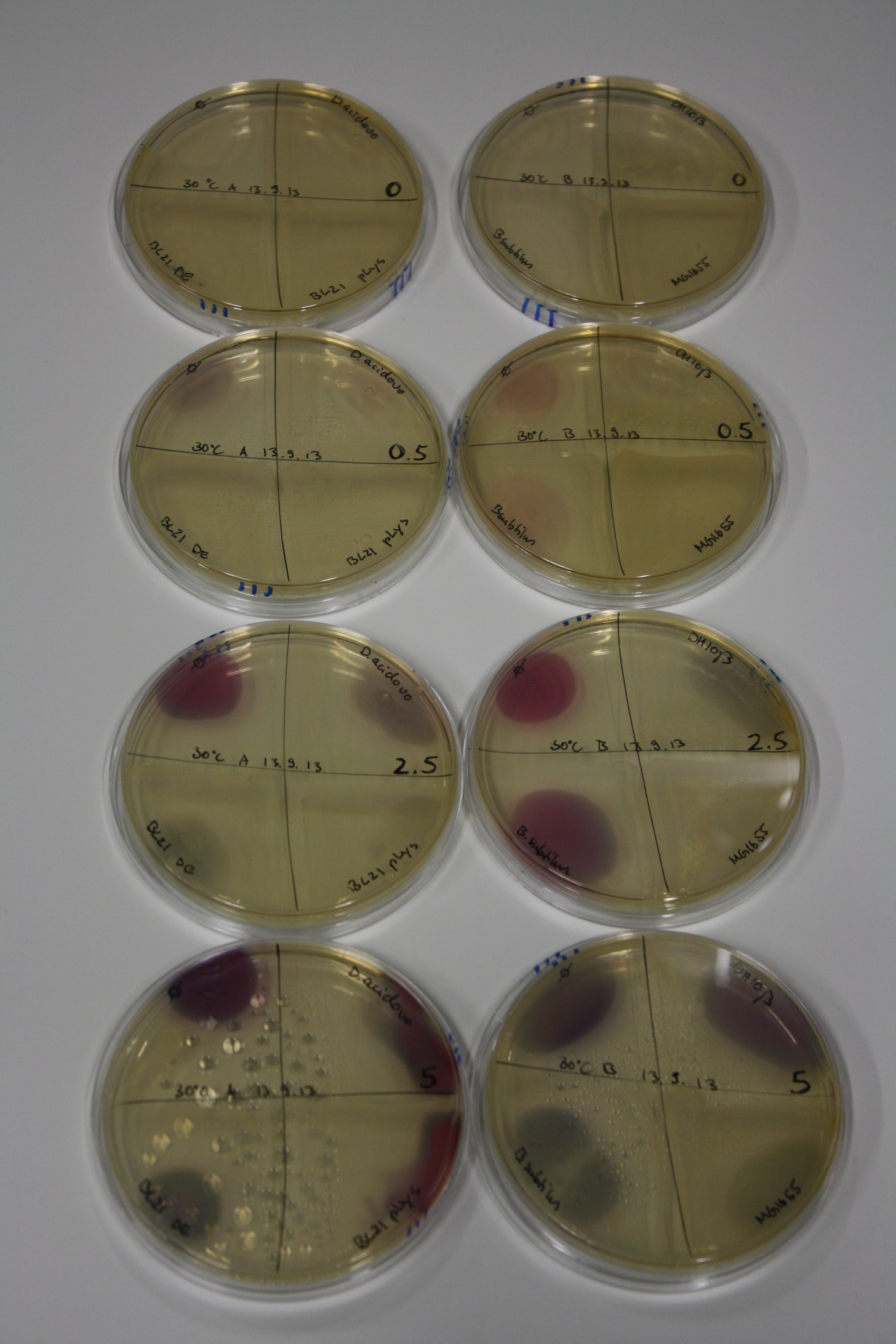
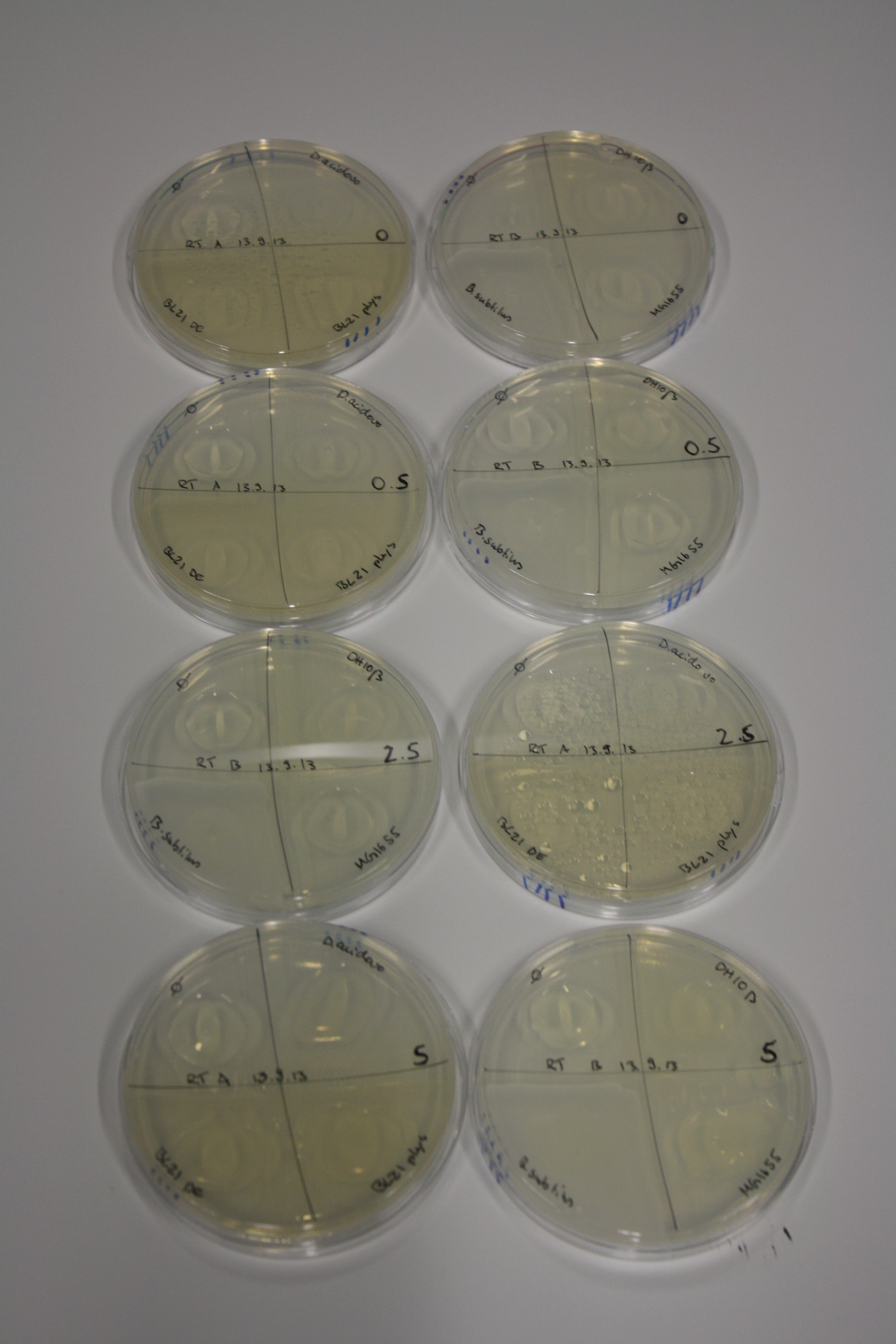
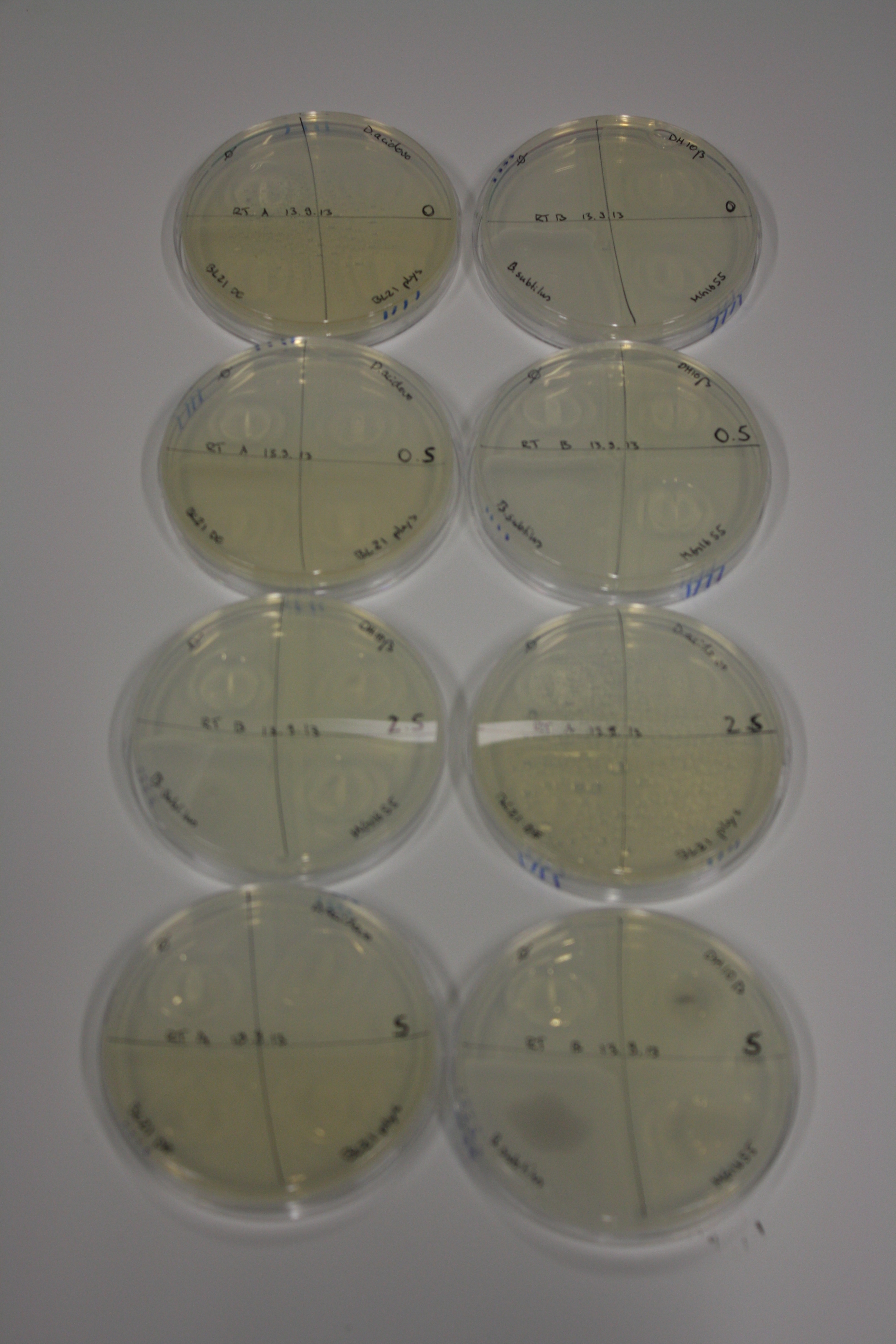
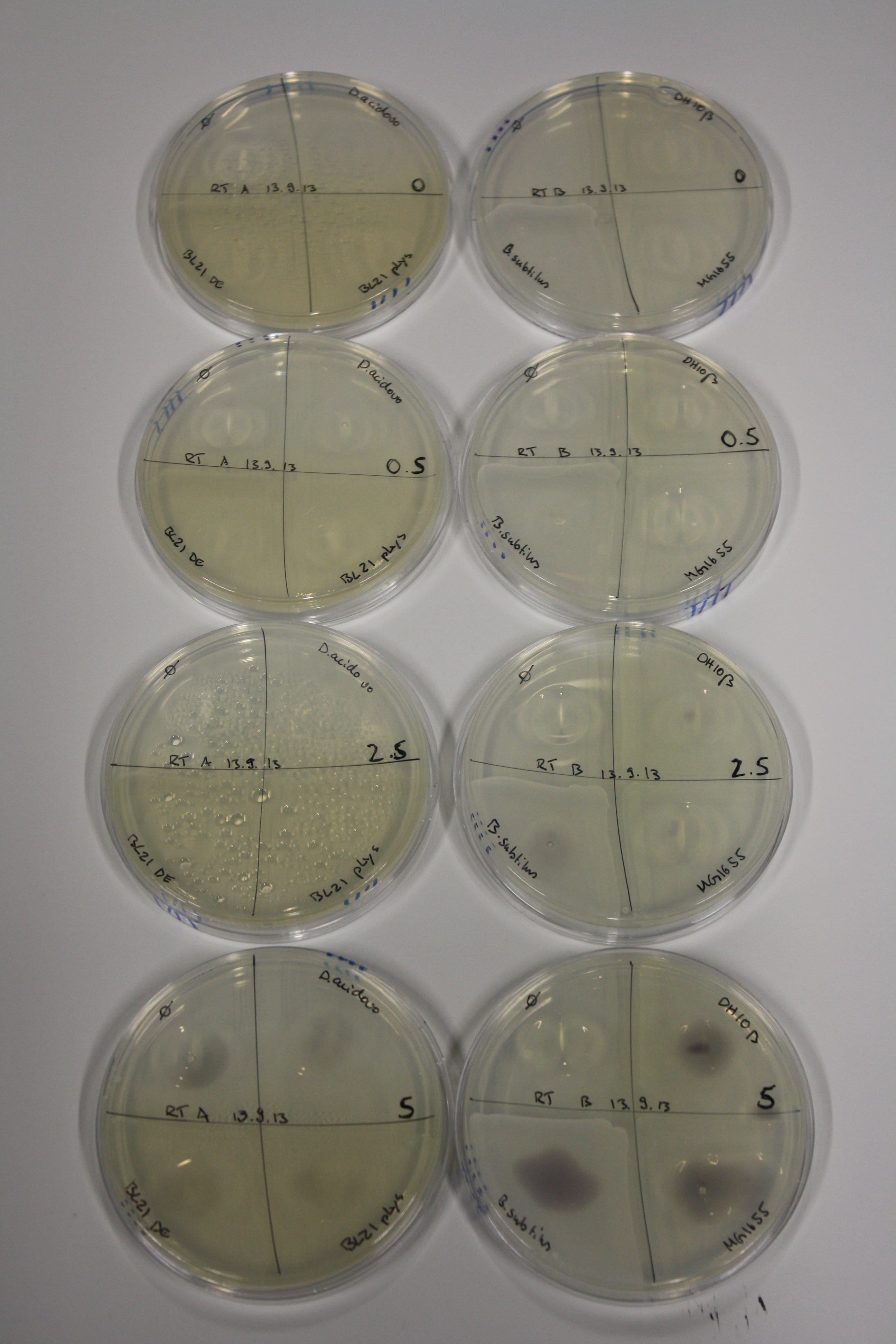
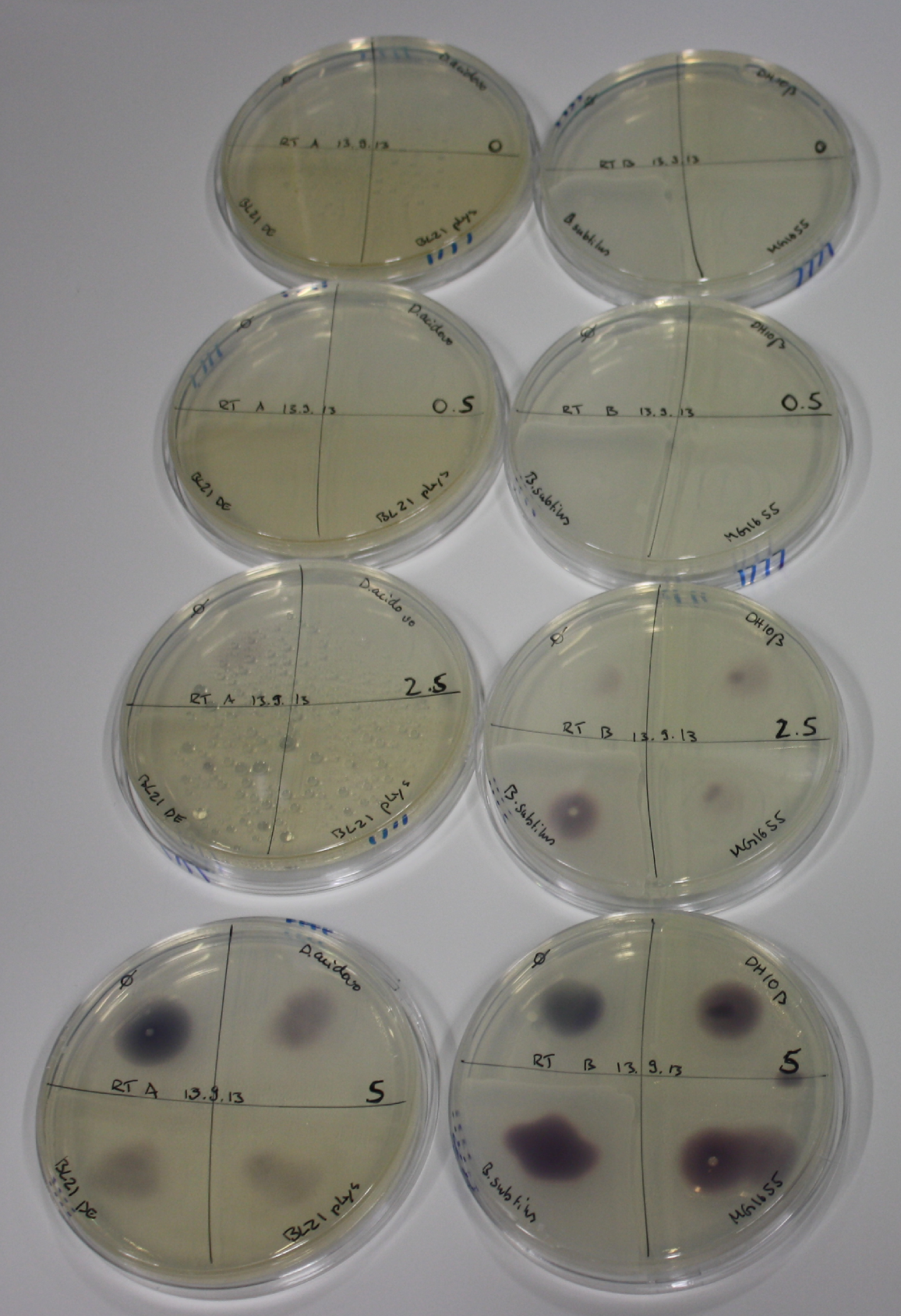
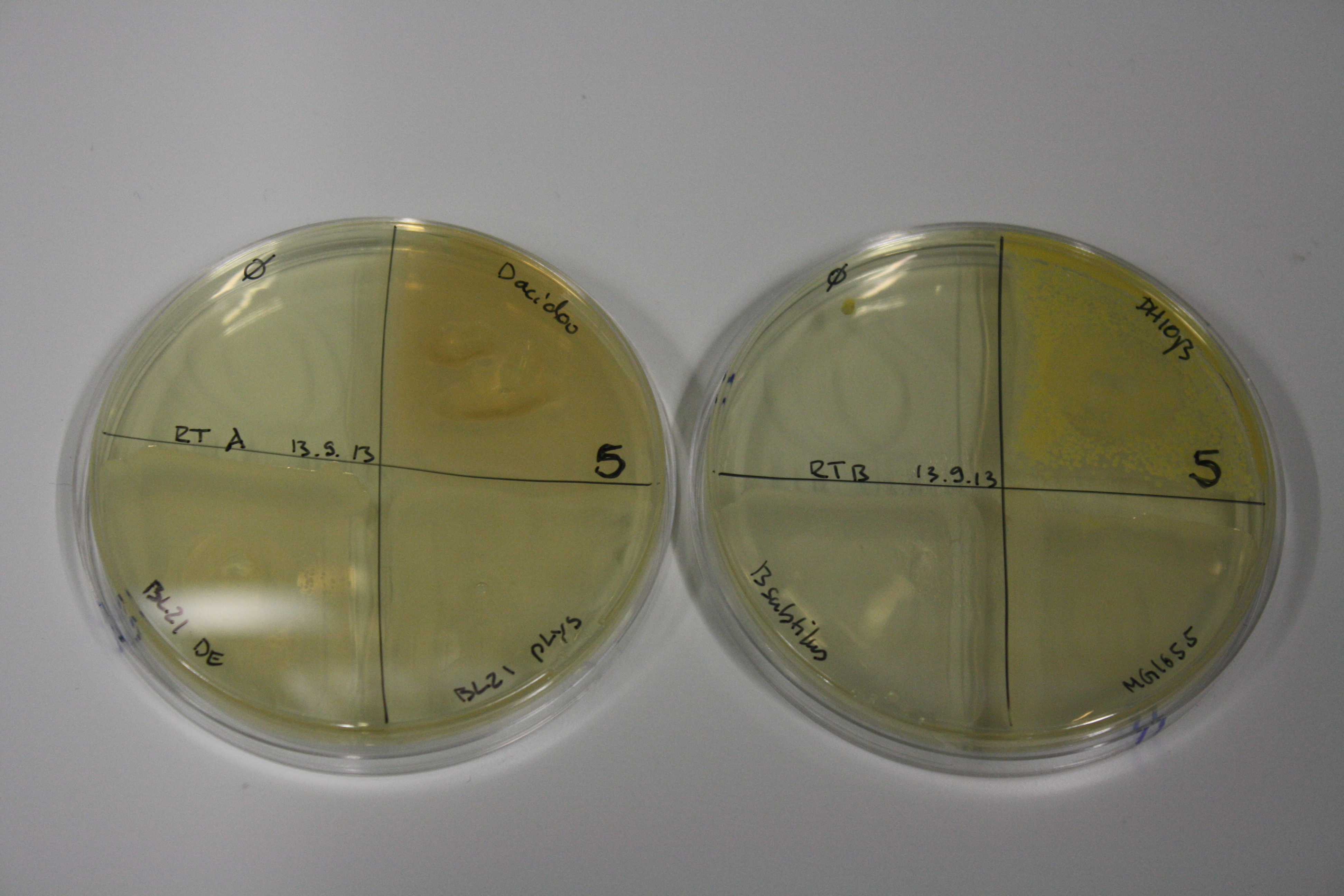
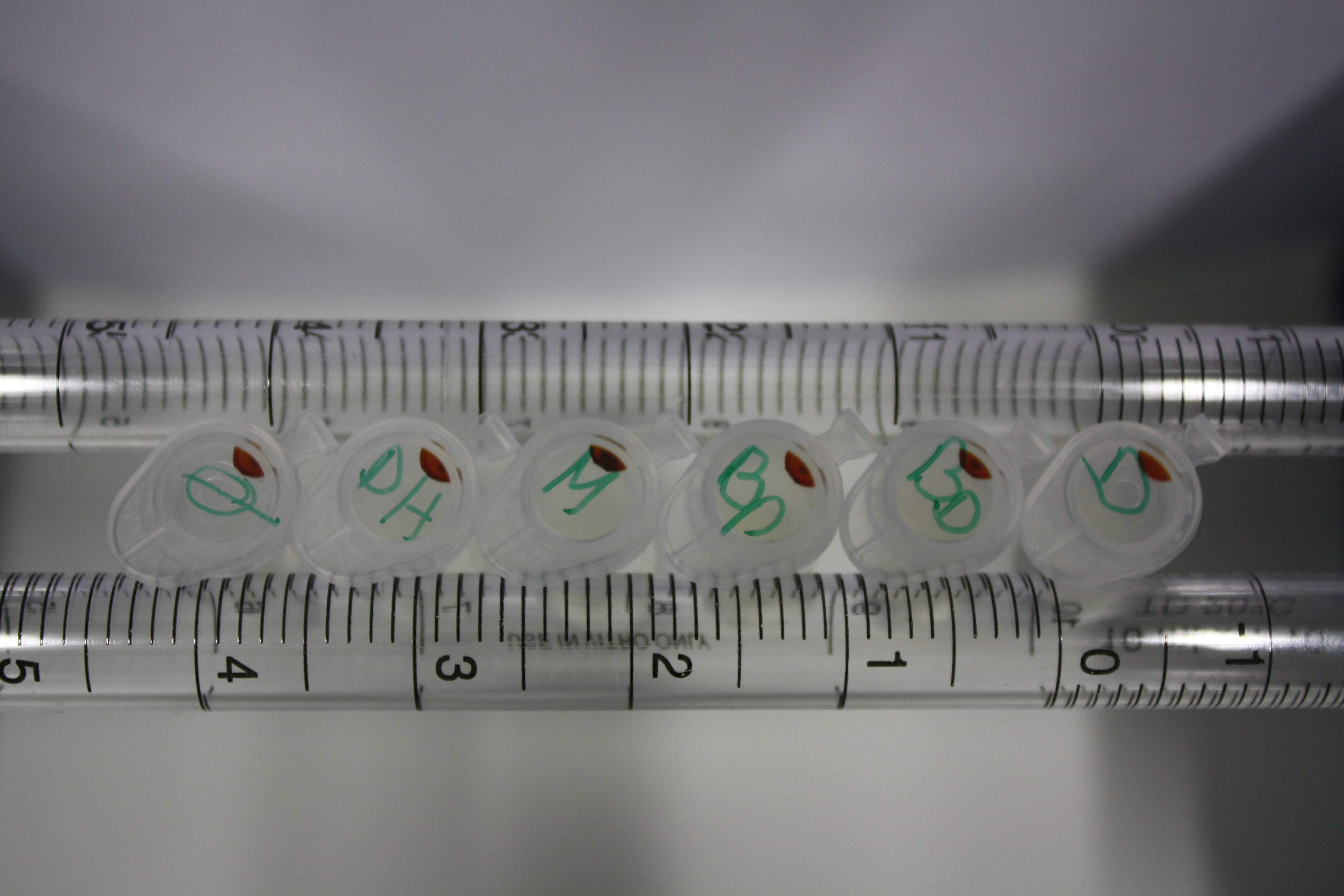
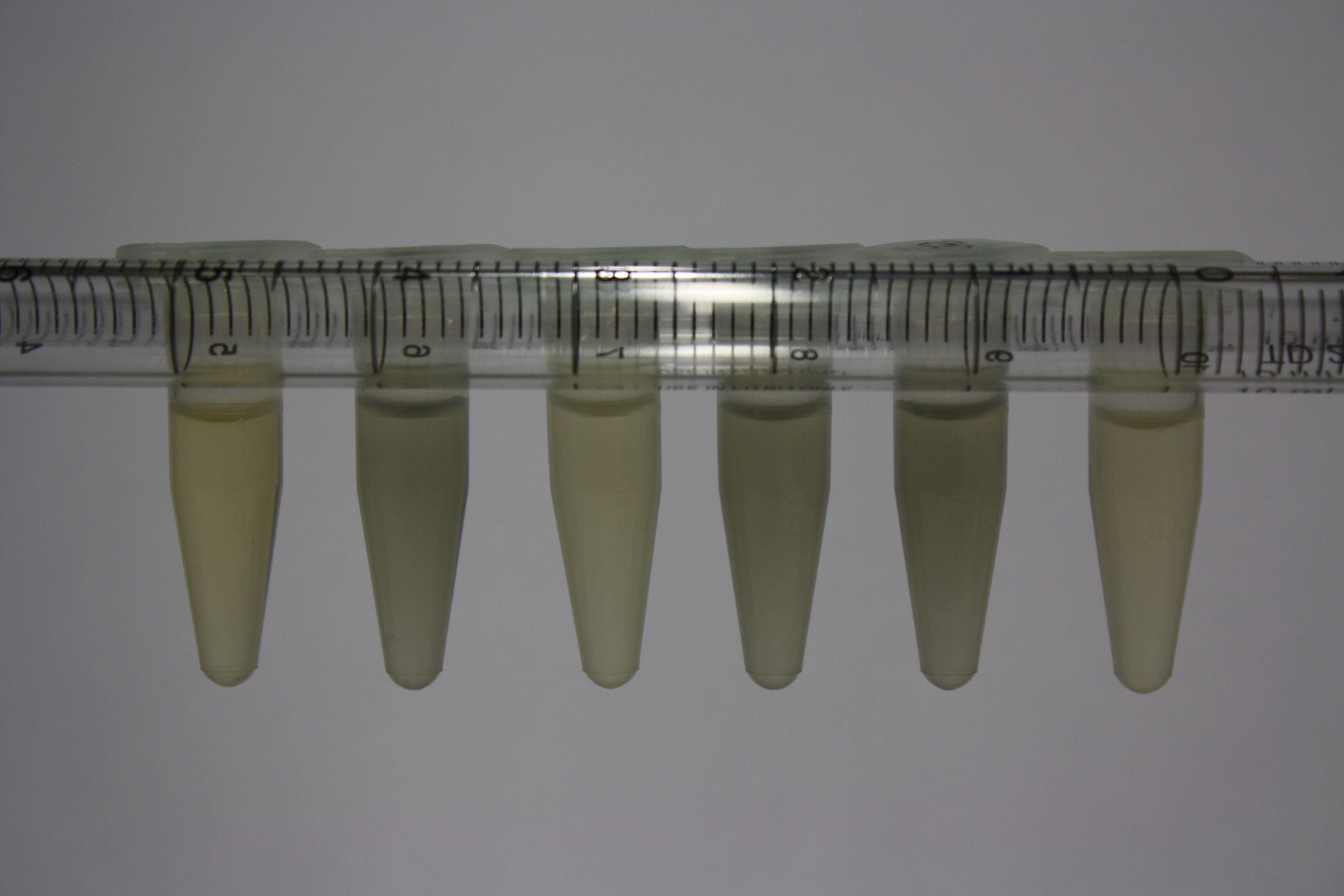
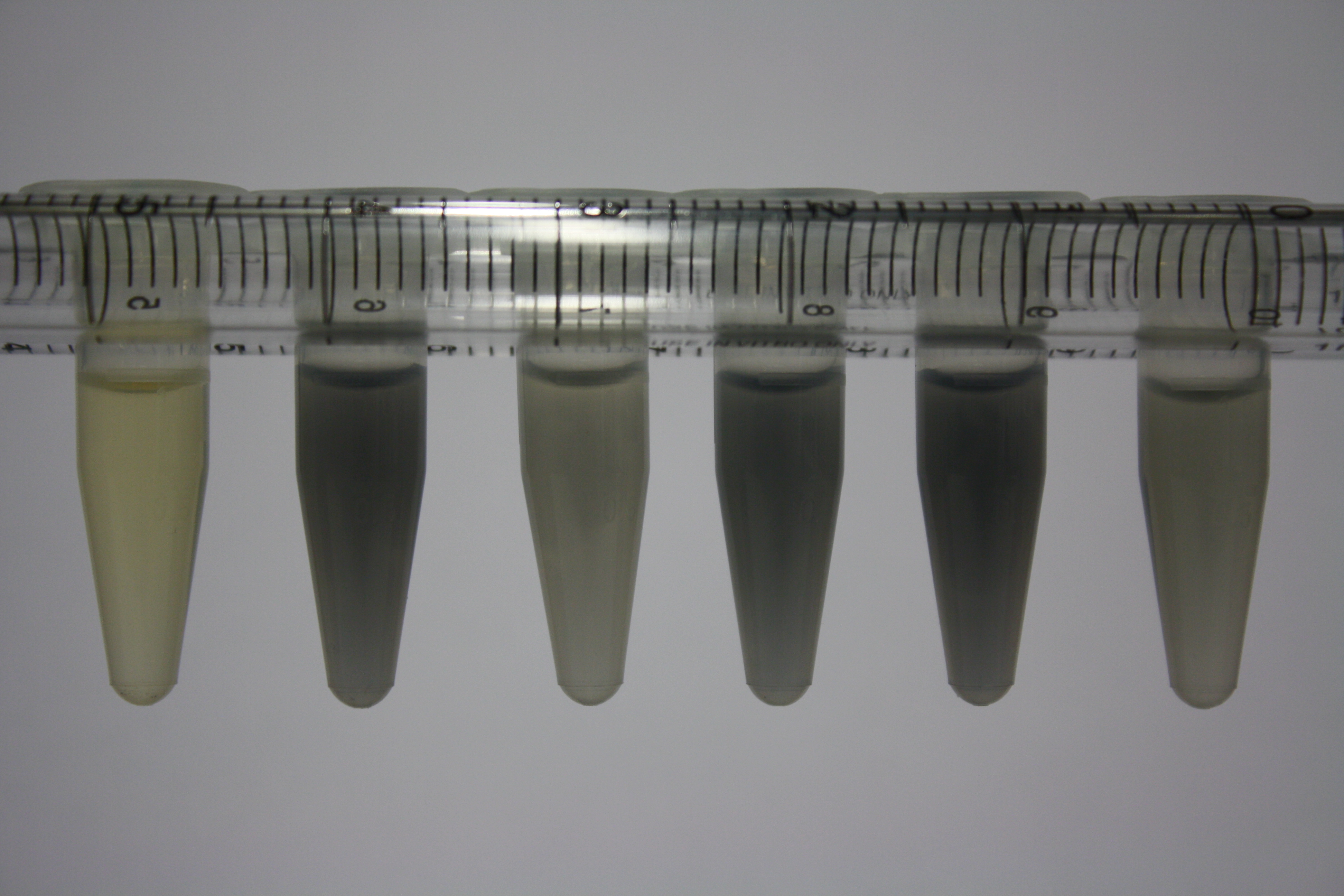
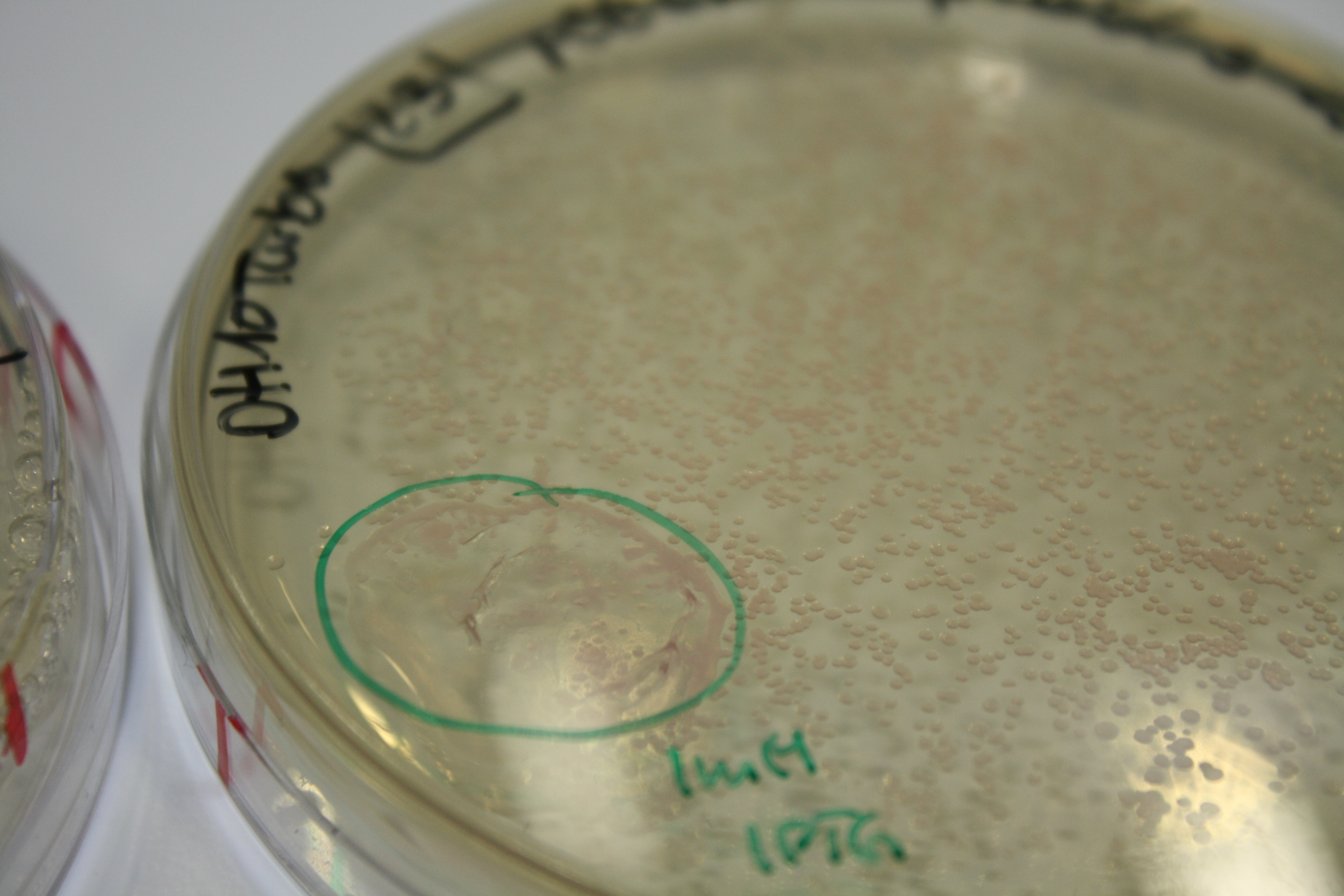
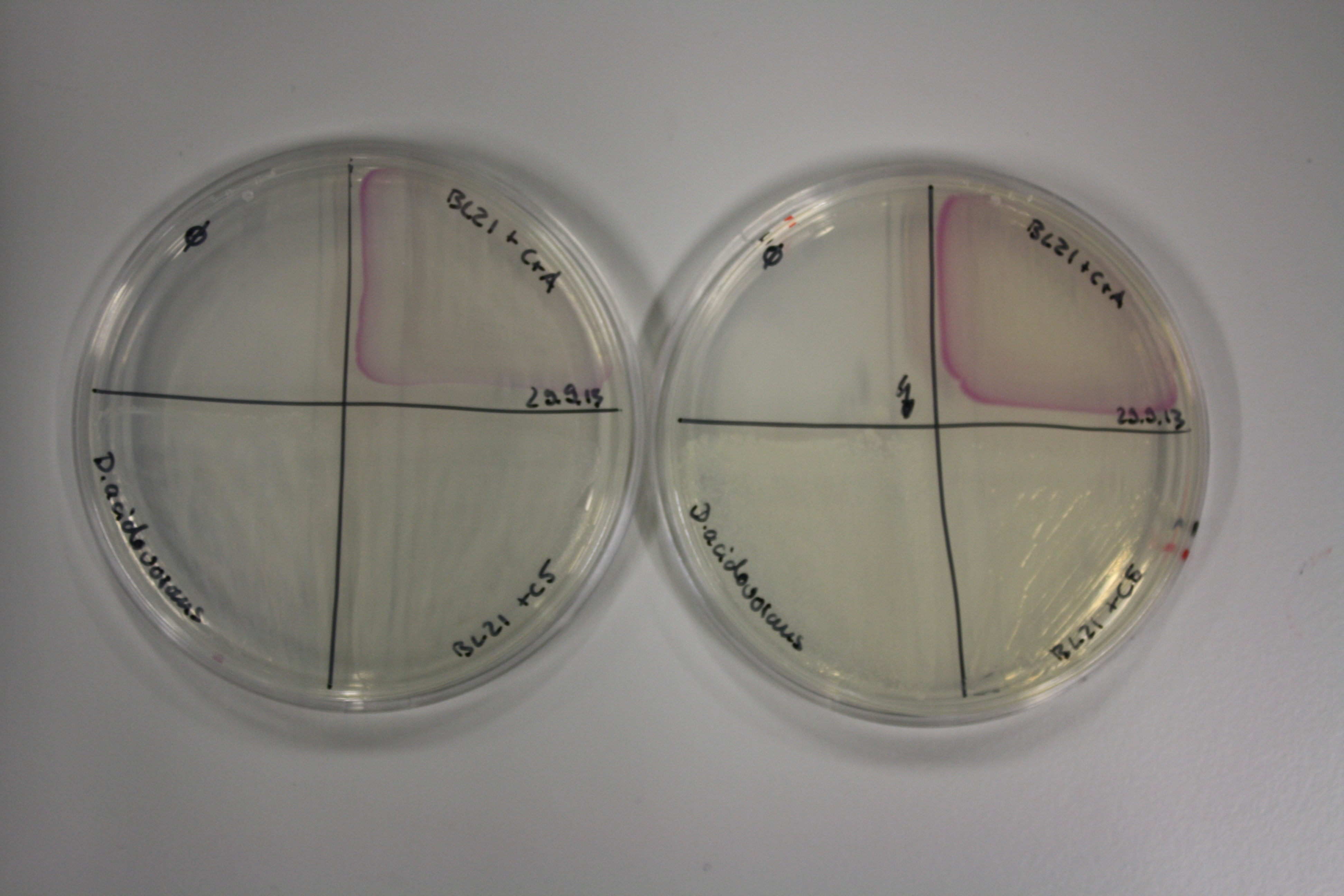
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">We optimized growth conditions of D. acidovorans and evaluated endogenous background precipitation of metall ions in the possible target E. coli strains E. coli DH10ß and BL21 DE3 following incubation over night on LB and ACM plates. D. acidovorans did not exceed E. coli, most probably due to insufficient cultivation time. When grown on LB plates, neither D. acidovorans nor E. coli showed any reactivity. </p> |
</div> | </div> | ||
</div> | </div> | ||
| Line 96: | Line 96: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 20</h1> | <h1>Week 20</h1> | ||
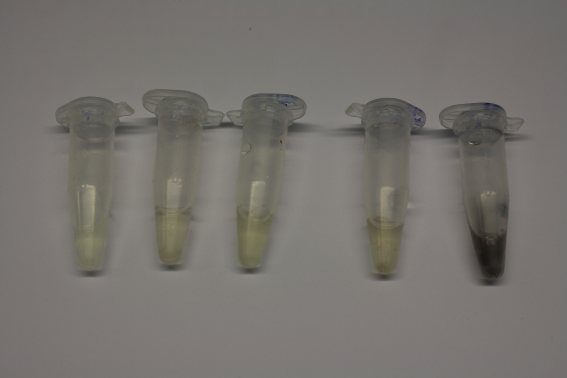
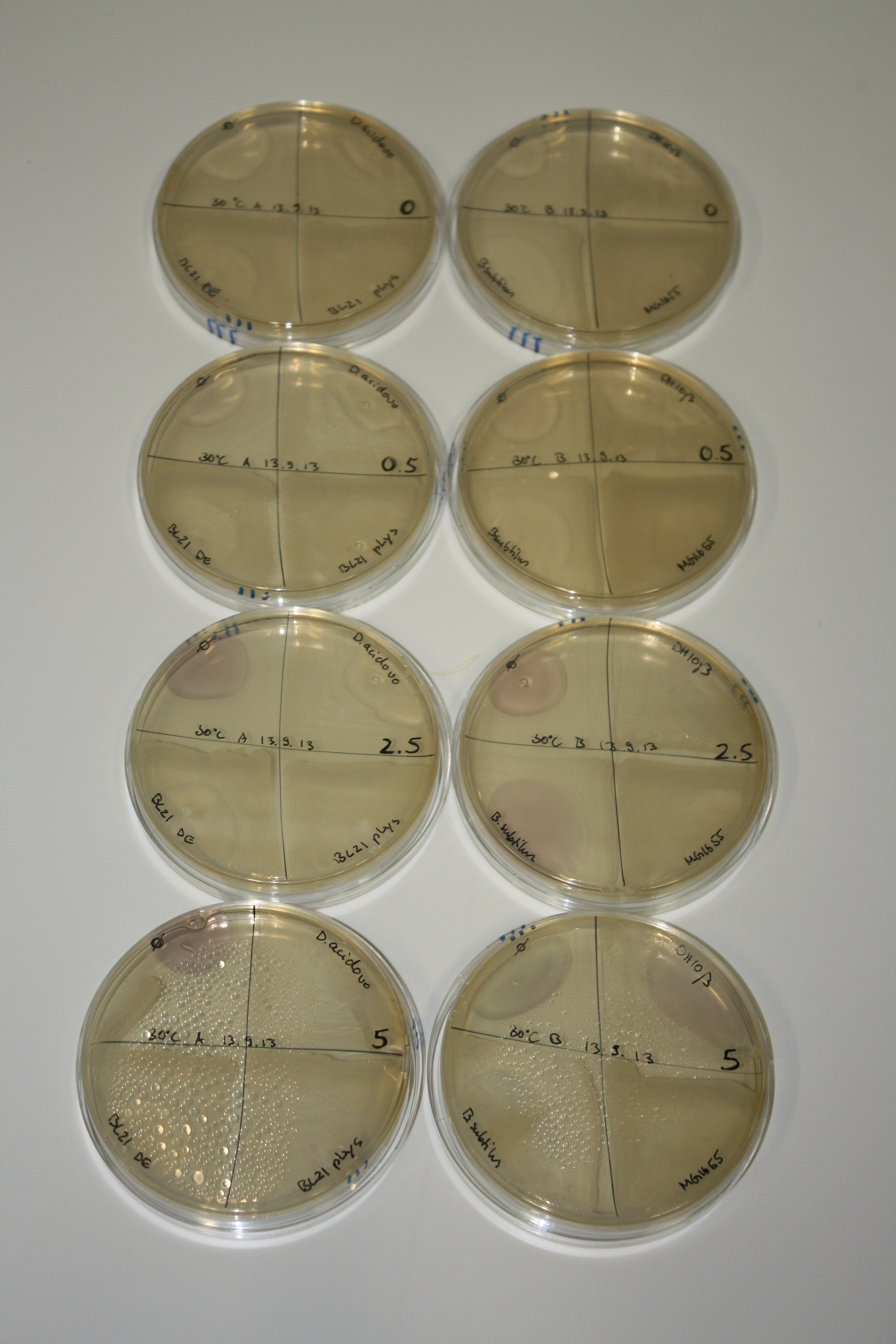
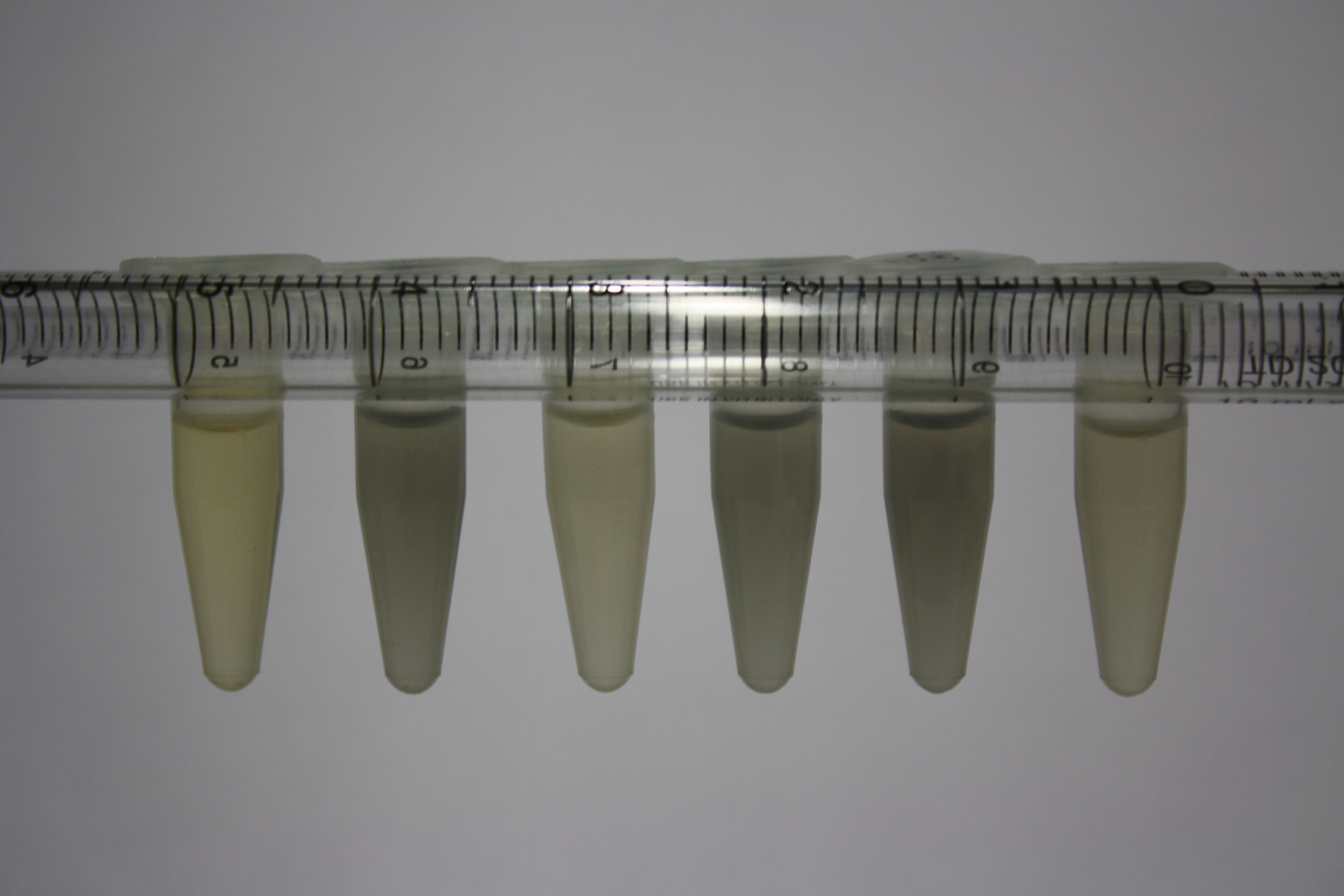
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">We continued optimized growth conditions of D. acidovorans and testing various E. coli strains for their endogenous capability to precipitate gold in order to identify the strain with the least background to be used as target strain after 2 and 3 days as well as grown at 30°C and room temperature. A cultivation at 30°C for 3 days was identified as optimal. We also started to establish purification of Delftibactin using HP20 resins and successfully verified presence of Delftibactin in the supernatant of D. acidovorans SHP-1. Additionally, we proved precipitation of gold by the purified Delftibactin and detected it by Micro-TOF File:20130911Malditof.pdf. Moreover, we triple-electroporated the final DelRest construct, the final MMCoA plasmid and the first promissing DelH clone into E. coli DH10ß. The Micro-TOF has to be repeated again next week. </p> |
</div> | </div> | ||
| Line 106: | Line 106: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 21</h1> | <h1>Week 21</h1> | ||
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">We repeated last week's Micro-TOF of the first promossing triple-clone, which did not show detectable expression of Delftibactin. </p> |
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 116: | Line 115: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 22</h1> | <h1>Week 22</h1> | ||
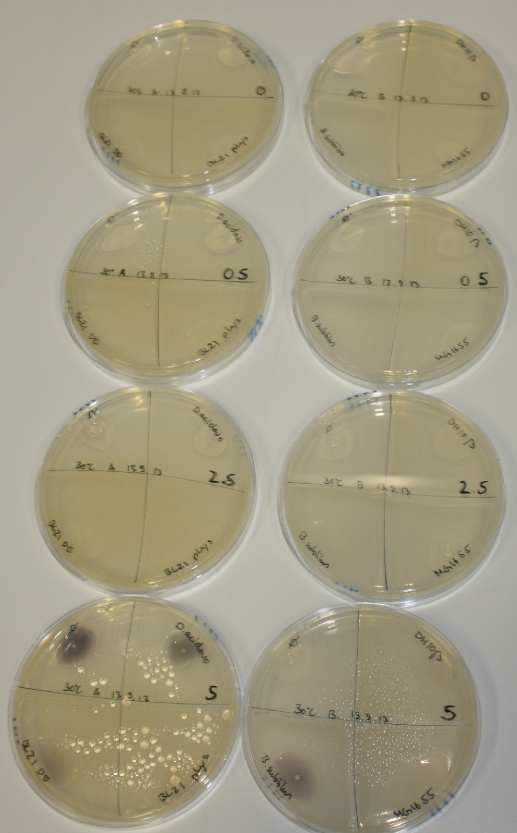
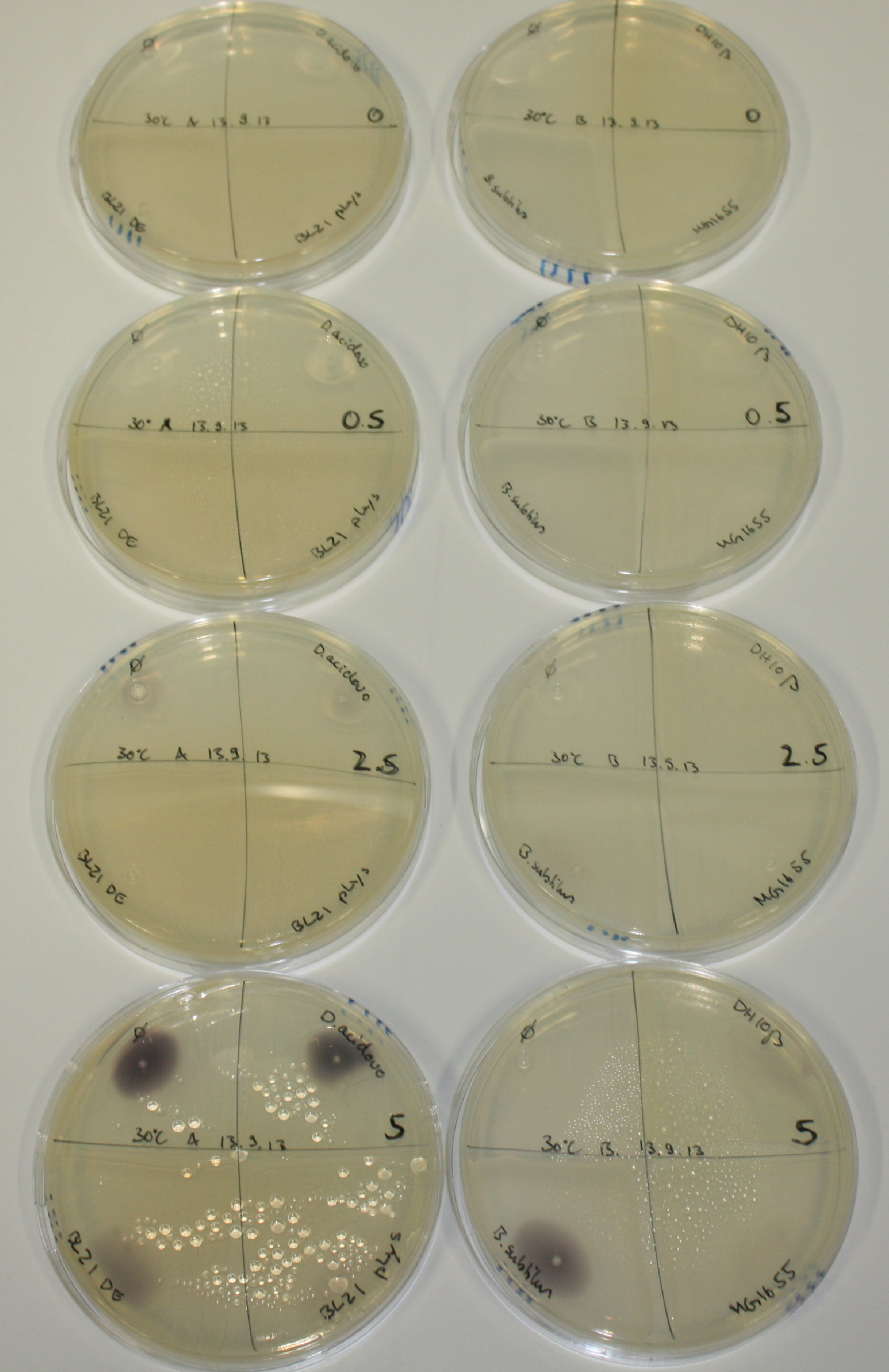
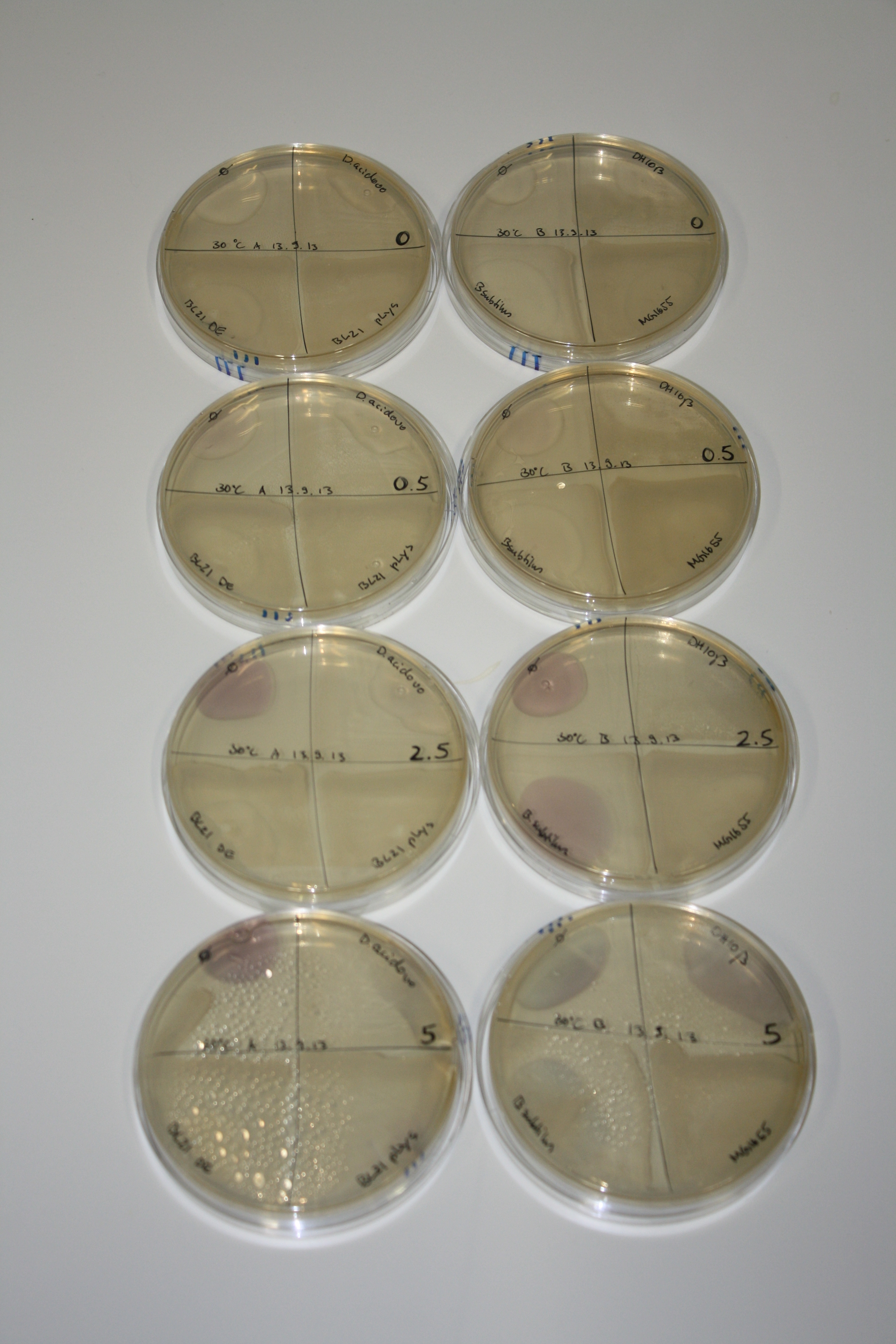
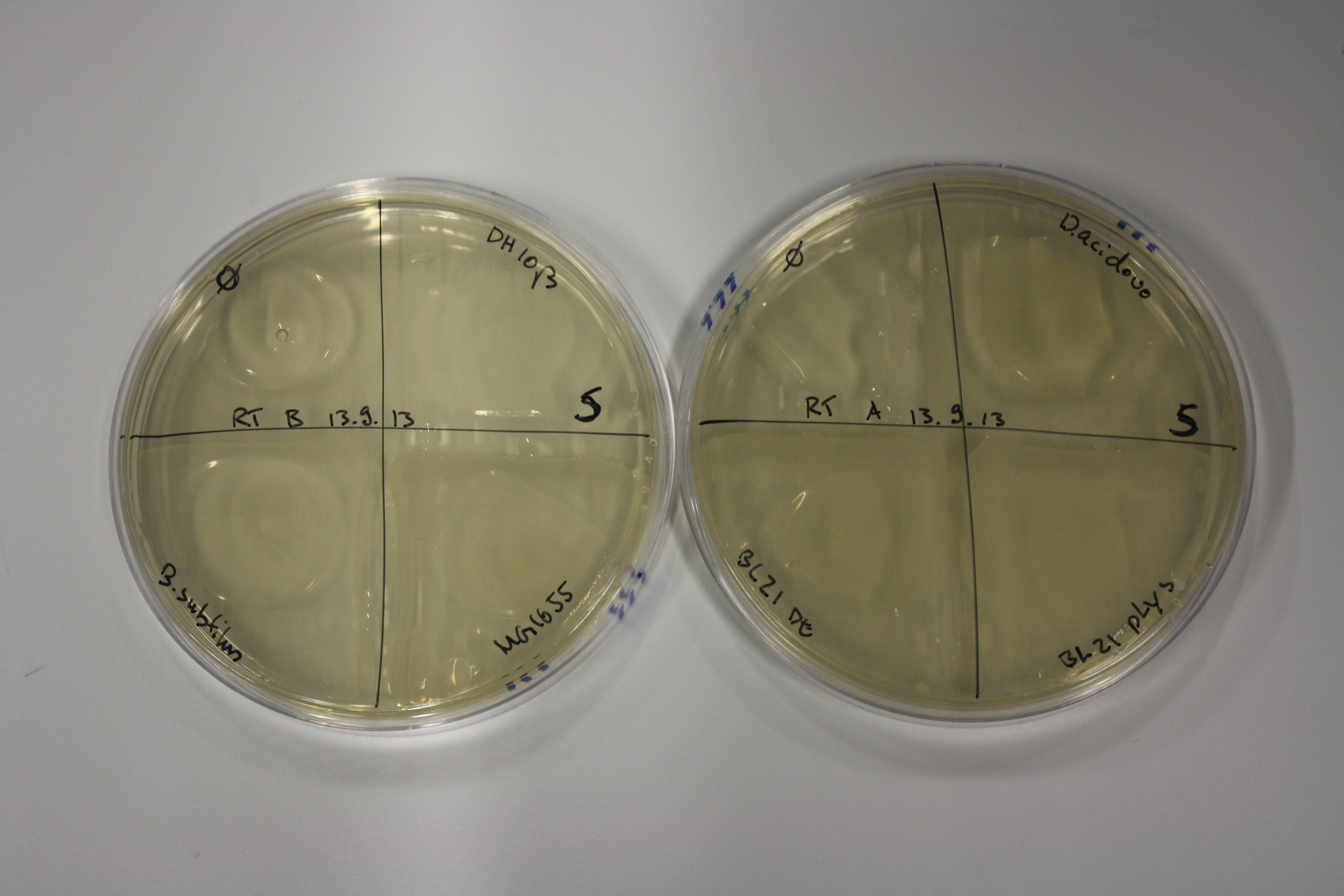
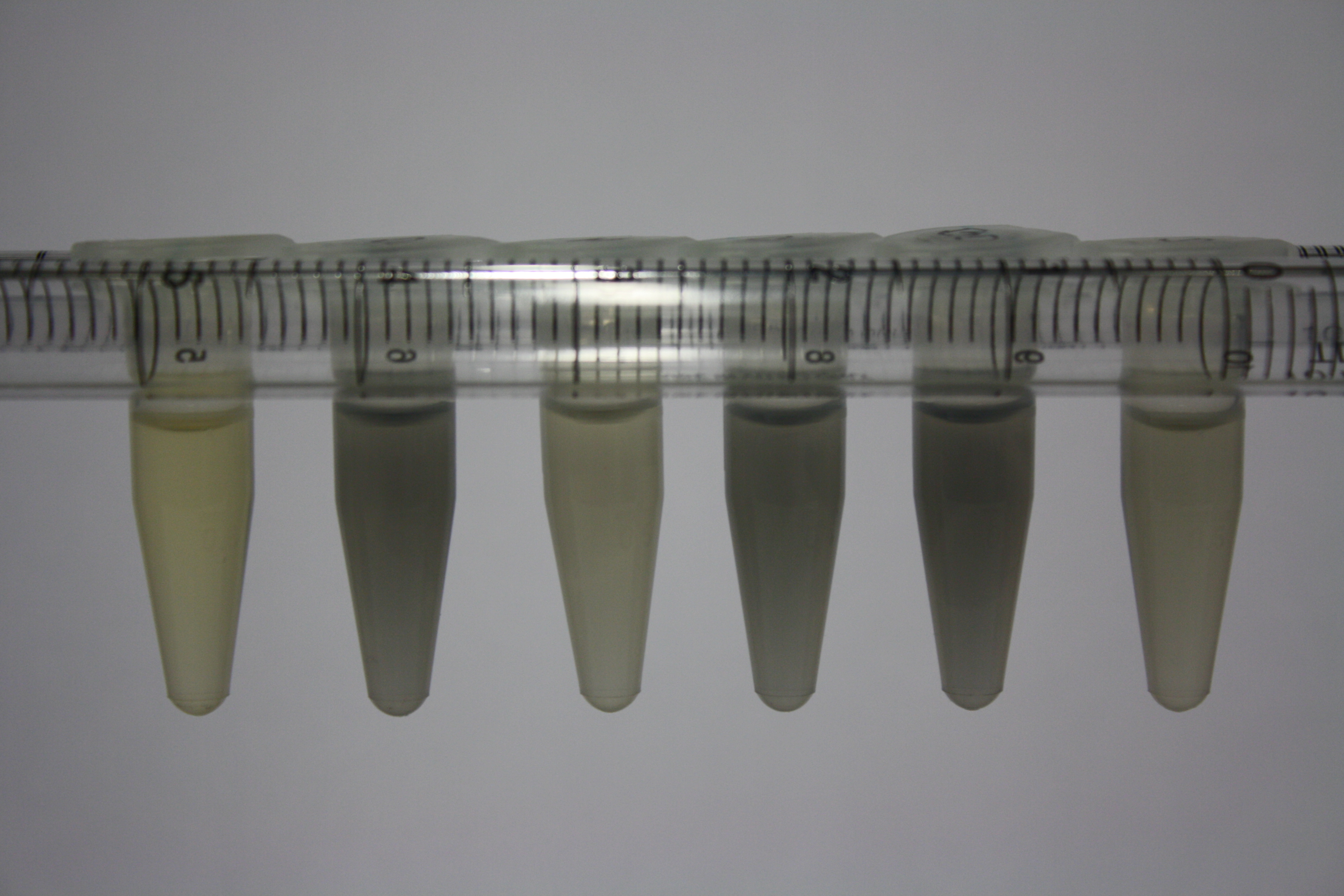
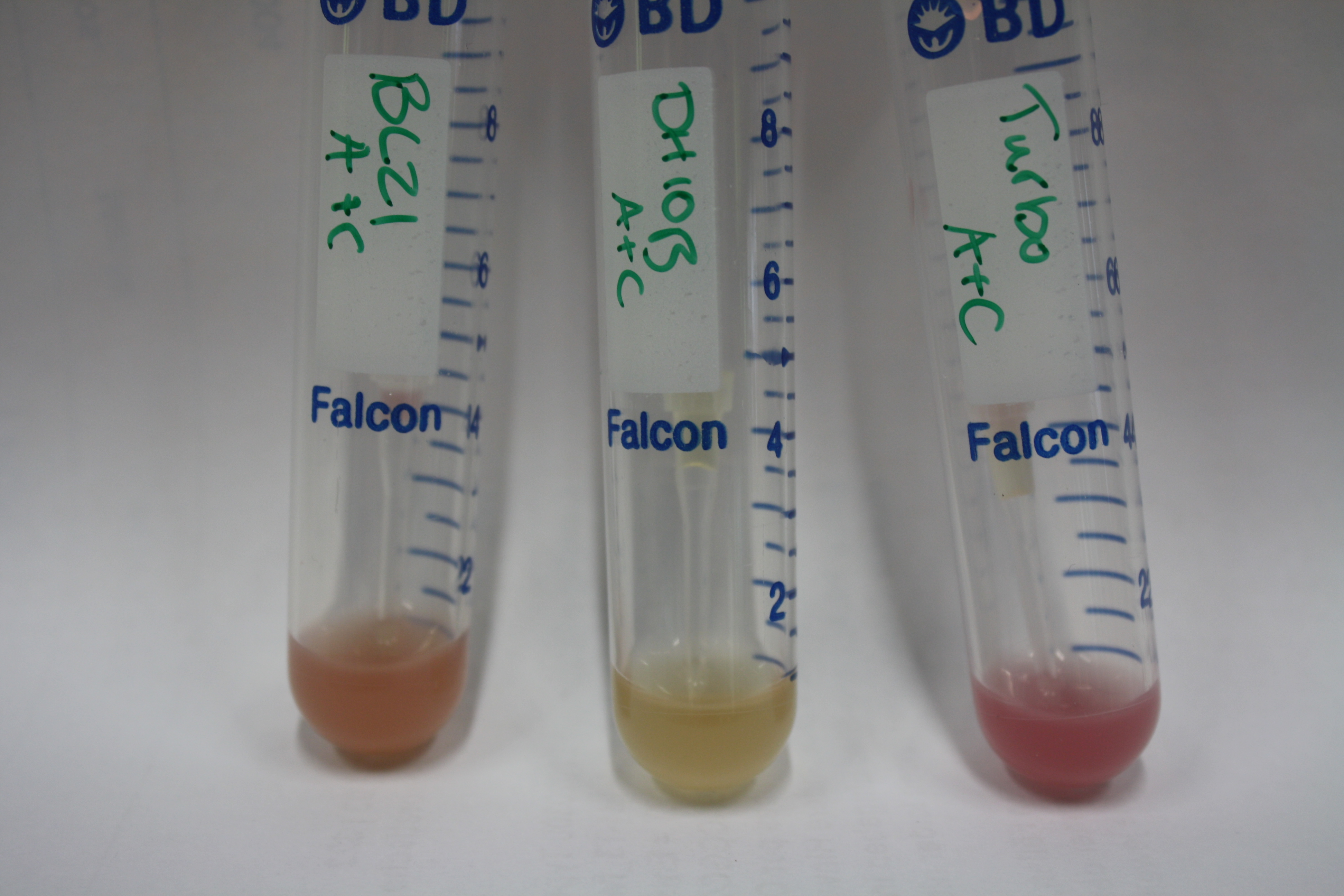
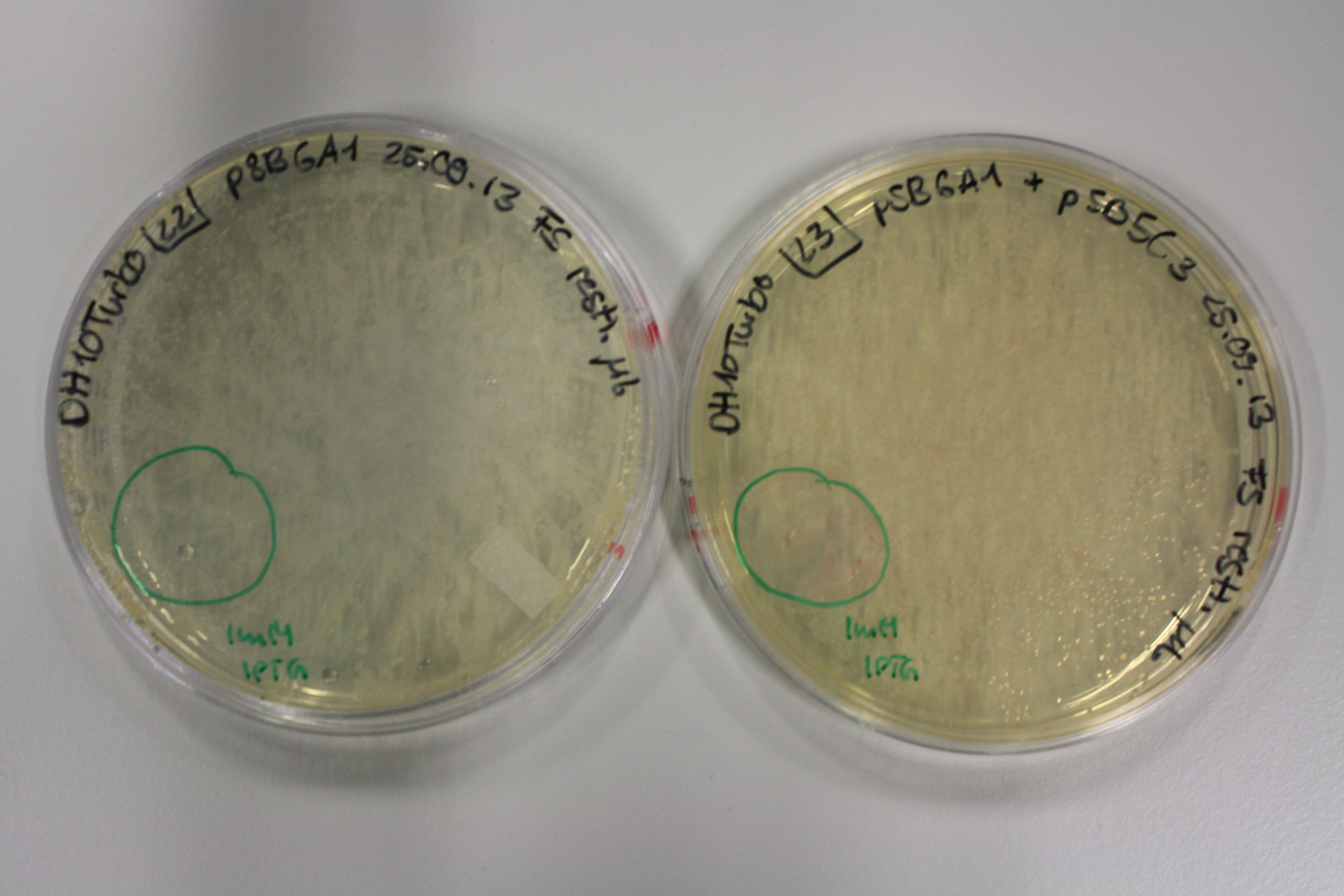
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">Possible E. coli target strains BL21 DE, DH10ß and NEB Turbo were analyzed for their background expression and indcibility. E. coli BL21 DE was identified as best of these three. It was electroporated with DelRest and pIK8.6 and of these, electrocompetent cells were prepared. </p> |
| - | + | ||
| - | + | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 127: | Line 124: | ||
<div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="carousel-caption scrollContent2" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
<h1>Week 23</h1> | <h1>Week 23</h1> | ||
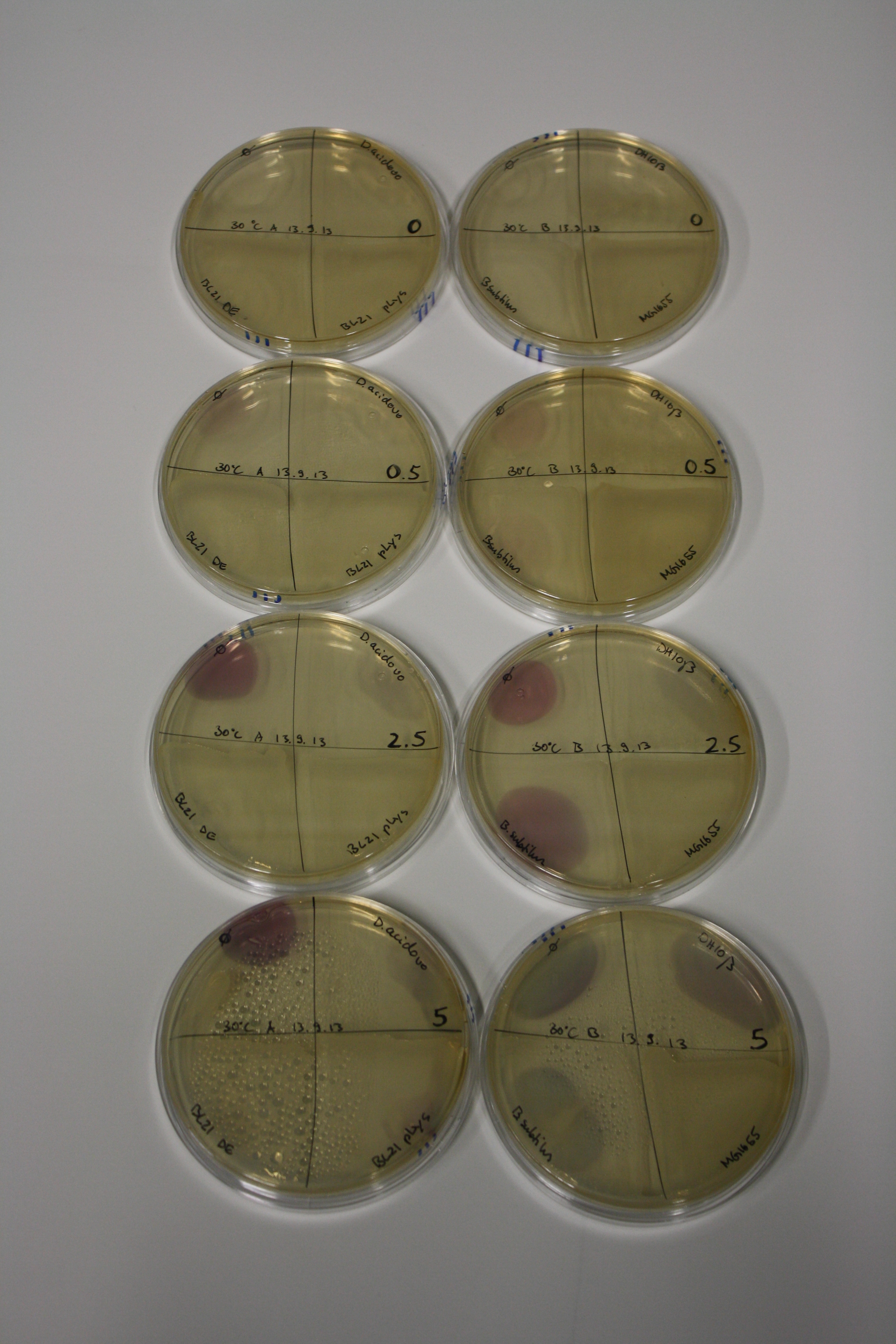
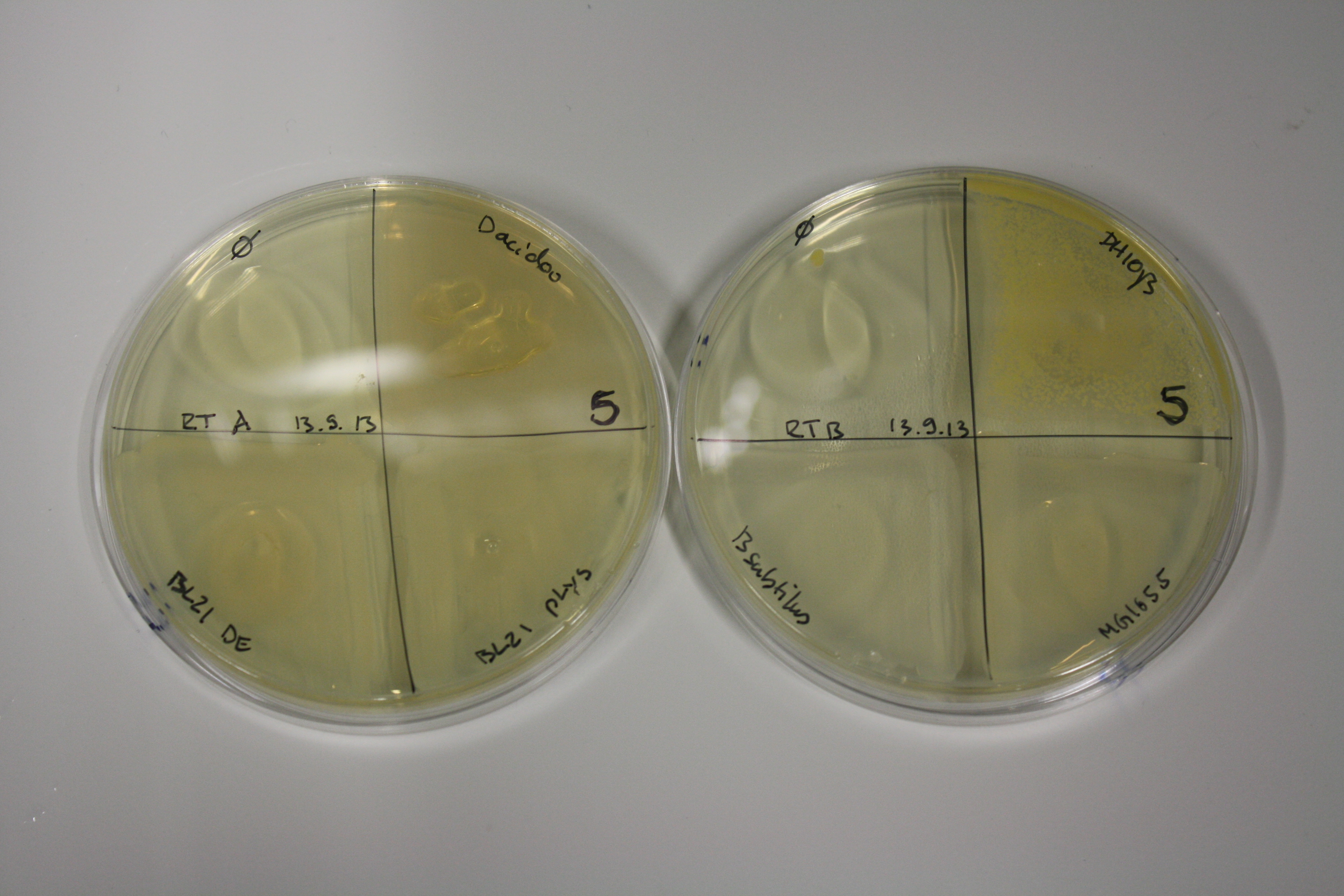
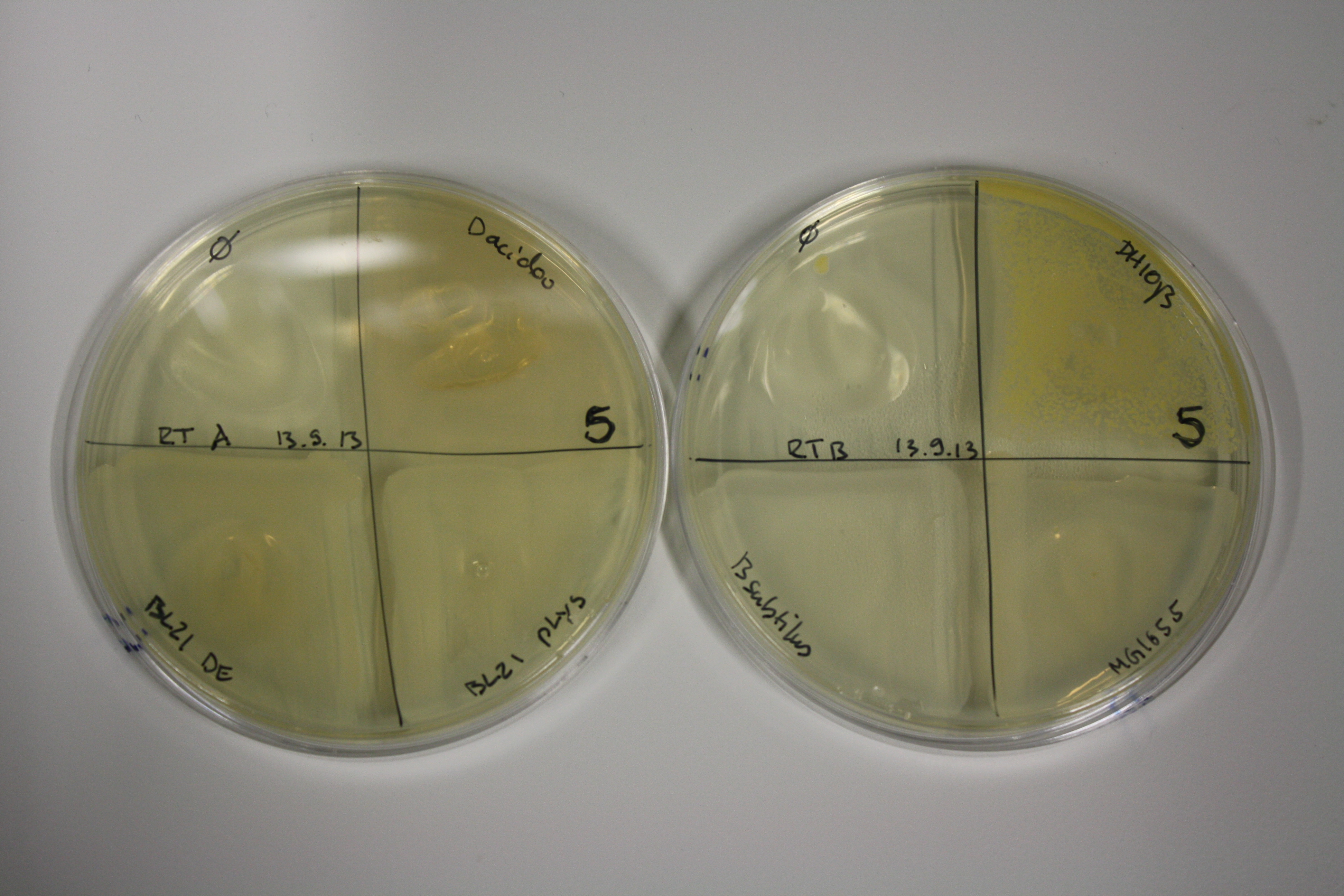
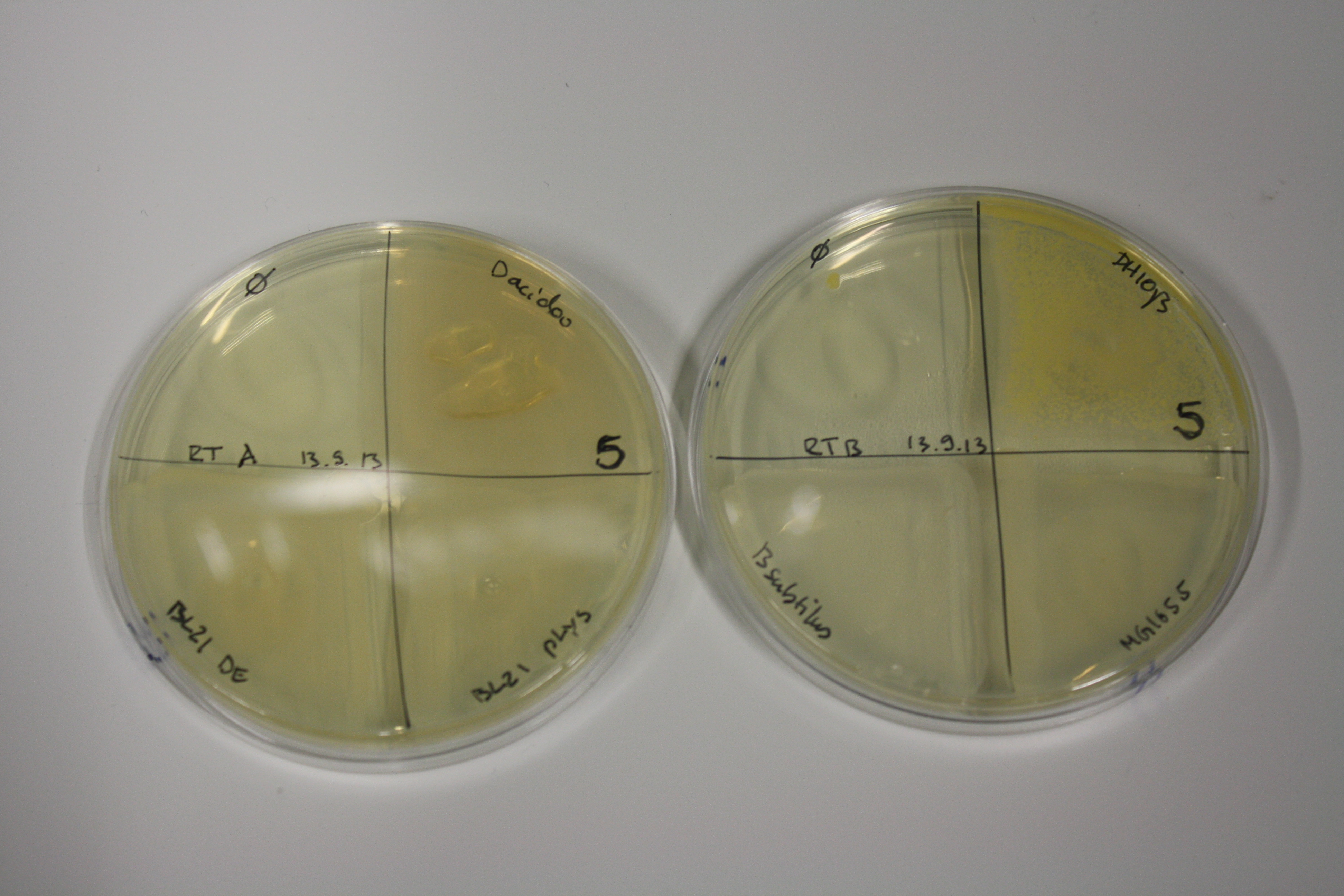
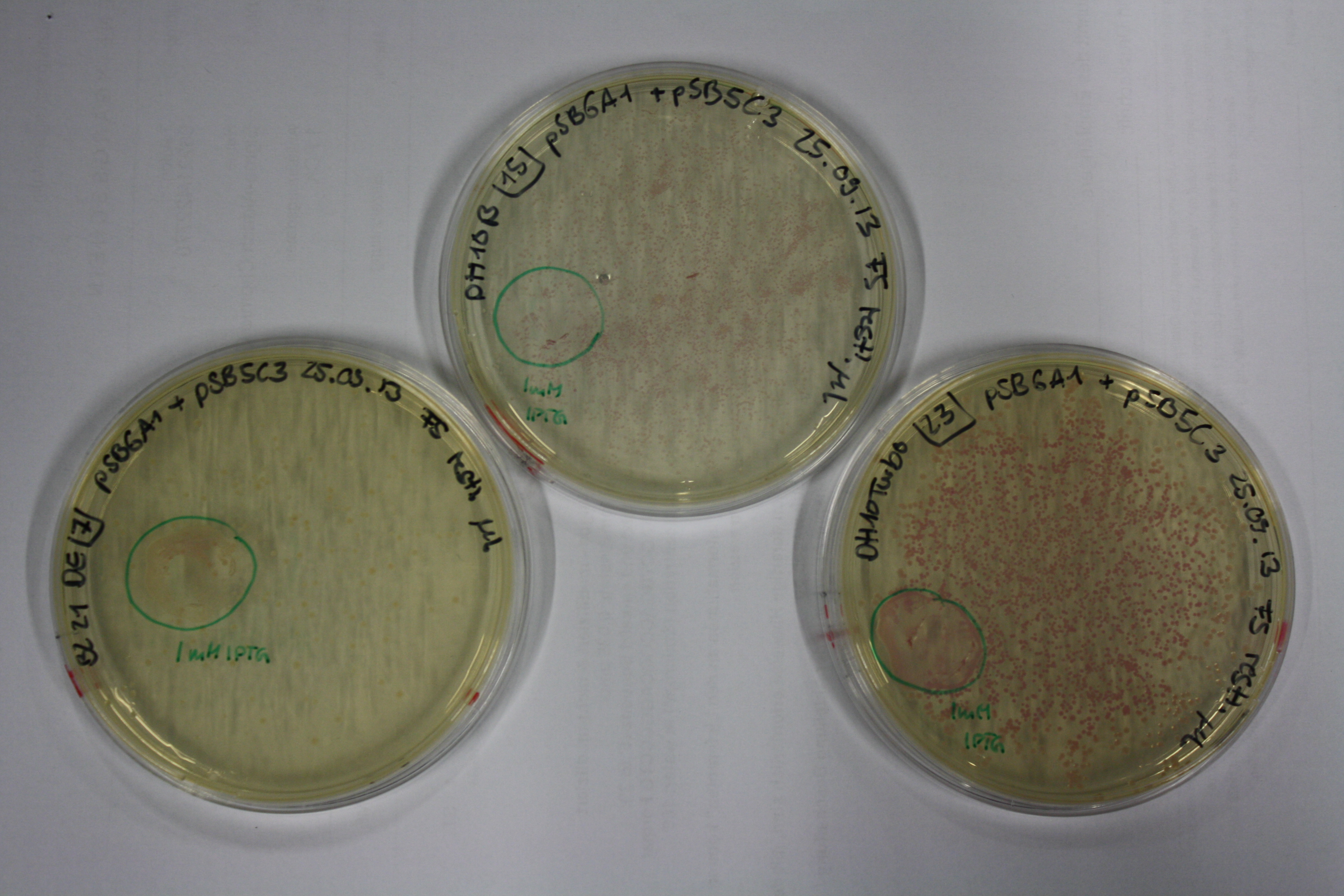
| - | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;"> | + | <p style="font-size:10pt; text-align:justify; position:relative; margin-left:6%;">E. coli BL21 DE + DelRest + pIK8.6 were elctroporated with the DelH clone C5, which harbors a amino acid substitution at the beginning of DelH. Its capability to precipitate gold from solution was analyzed on ACM plates following induction by IPTG. Due to a contamination, the results were inconclusive. The production of Delftibactin was accessed by Micro-TOF. </p> |
<p><a class="btn btn-large btn-primary" href="#">Browse gallery</a></p> | <p><a class="btn btn-large btn-primary" href="#">Browse gallery</a></p> | ||
</div> | </div> | ||
| Line 253: | Line 250: | ||
<div> | <div> | ||
<ul class="nav nav-tabs"> | <ul class="nav nav-tabs"> | ||
| - | <li class="active" | + | <li class="active"><a href="#b19" data-toggle="tab">Lab book</a></li> |
| - | + | ||
</ul> | </ul> | ||
</div> | </div> | ||
| Line 260: | Line 256: | ||
<div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<div class="tab-pane" id="b19"> | <div class="tab-pane" id="b19"> | ||
| Line 313: | Line 302: | ||
<div> | <div> | ||
<ul class="nav nav-tabs"> | <ul class="nav nav-tabs"> | ||
| - | <li class="active" | + | <li class="active"><a href="#b21" data-toggle="tab">Lab book</a></li> |
| - | + | ||
</ul> | </ul> | ||
</div> | </div> | ||
<div class="jumbotron"> | <div class="jumbotron"> | ||
<div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<div class="tab-pane" id="b21"> | <div class="tab-pane" id="b21"> | ||
| Line 372: | Line 352: | ||
<div> | <div> | ||
<ul class="nav nav-tabs"> | <ul class="nav nav-tabs"> | ||
| - | <li class="active" | + | <li class="active"><a href="#b23" data-toggle="tab">Lab book</a></li> |
| - | + | ||
</ul> | </ul> | ||
</div> | </div> | ||
<div class="jumbotron"> | <div class="jumbotron"> | ||
<div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | <div class="nav navbar" data-spy="scroll" data-target="#navbarExample" data-offset="0"> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<div class="tab-pane" id="b23"> | <div class="tab-pane" id="b23"> | ||
Revision as of 11:57, 4 October 2013


Delftibactin. Bringing it all together.
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet.
Methods:
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet. Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet.Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. .
 "
"