Team:NTU-Taida/Project/Background
From 2013.igem.org
Contents |
Bacterial infection
Bacterial infection is the invasion of bacteria into one’s body. They reproduce and multiply themselves, causing disease by local cellular injury, secretion of toxins, or antigen-antibody reaction in the host. The spectrum of bacteria changes with time and the introduction of antibacterial agent. Nowadays, the emerging issue in this field is the appearance of multiple drug-resistant (MDR) bacteria and extensive drug-resistant (XDR) bacteria. Yet the development of resistant strains of bacteria could be limited by the judicious use of antibiotics. In order to use the appropriate drug, knowing the antibiotic pattern of the invading bacteria is of importance. The routine process to identify causative organism in hospital, culturing and sensitivity testing for a specific antibiotic, is found to be sensitive.
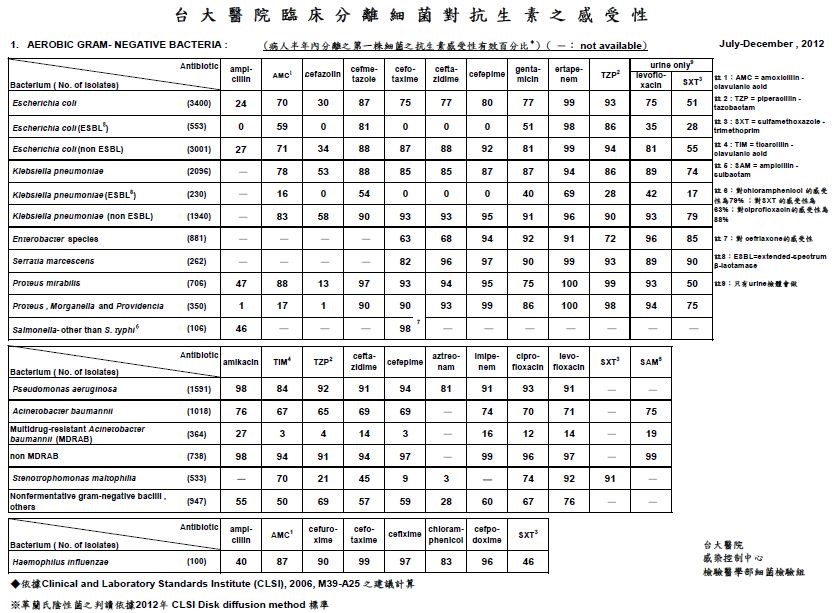
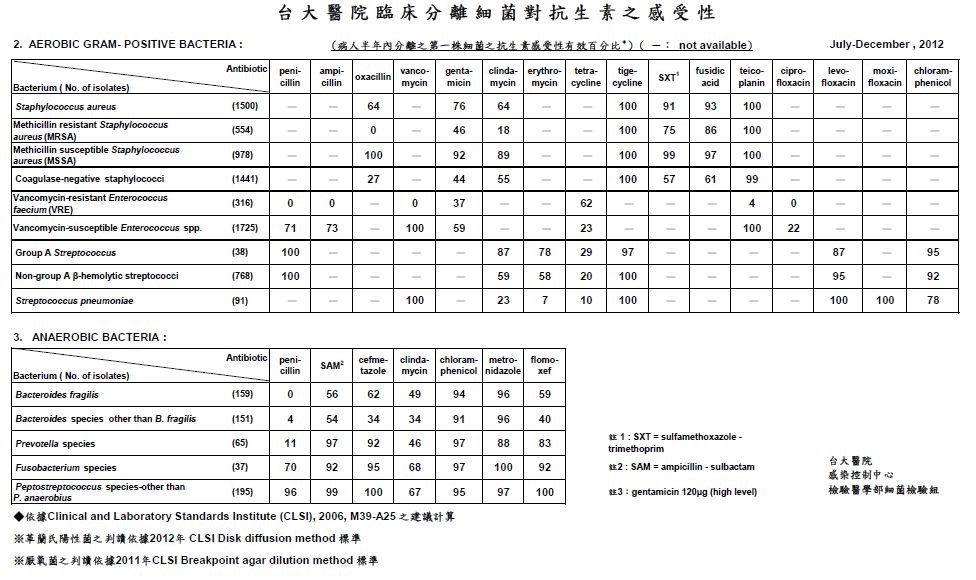
Bacterial infection may be further classified by where the infection occurs, into community-acquired infection and nosocomial infection. The strains of bacteria, infection site, and epidemiological transmission pathway all differ between them. Nosocomial infection is of increased importance. MDR or XDR are present in hospital environments such as MRSA (Methicillin-resistant Staphylococcus aureus) and Acinetobacter. The Table below is the susceptibility of antibiotics of clinical separated bacteria from NTUH (National Taiwan University Hospital) at 2012. Urinary tract infections, pneumopathy, and infections of surgery site are most common because of the formation of biofilm on the surface of catheters, endo-tubes, etc. What’s worse, because of the drug-resistance, diagnosis and treatment of bacterial nosocomial infection become a serious problem. The identification of bacteria and new drugs is required.
Quorum Sensing System in Gram Positive Bacteria
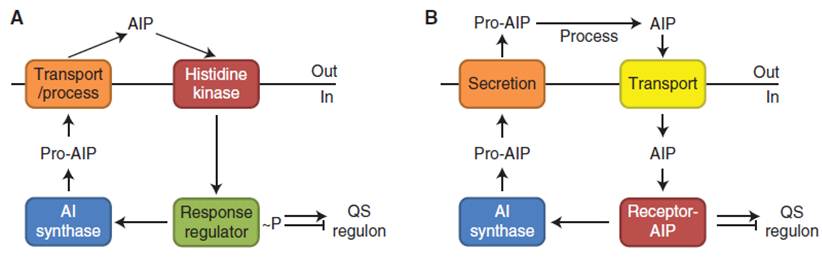
Gram-positive bacteria, characterized with a relatively thick layer of peptidylglycan, generally use peptides as quorum sensing molecules. These peptides are called autoinducing peptides (AIPs); when produced by specialized AI synthase, they can be either transported from(outside?) the cells through a specific membrane-bound transporter, or processed and secreted directly from the membrane. A high concentration of extracellular AIPs indicates high cell density. By binding to its cognate membrane-bound sensor kinase or intracellular receptor, the quorum sensing signal is transmitted back and spread to other bacterial cells, creating an inter-cellular communication network. The cytoplasmic response regulator, which controls the downstream virulence factors and AI(AIP?) synthase, is phosphorylated by the histidine kinase receptor upon extracellular binding of AIPs, or is activated by the intracellular receptor-AIP complex itself (Figure 1).
Together from a module-based point of view, the whole process of quorum sensing includes production, detection and response. Collectively these modules are termed a two-component system.
Clinically important Gram-positive bacteria include Staphylococcus aureus and Streptococcus pneumoniae, responsible for skin and respiratory infections respectively. S. aureus uses the agr as its quorum sensing system.
Routine Identification
Laboratory medicine (see also Visit[1]) plays a crucial role in hospital and the identification of infecting bacteria are big part of it. According to the visit to professor Po-Ren Hsueh, a visiting staff in the department of laboratory medicine in NTU hospital, the routine identification of bacteria and sensitivity tests are mainly through the incubation in the plate. The method through mass spectrometry, having substantial advancement nowadays, can quickly excite proteins in bacteria, match in database and identify bacteria in 5 min, while methods via bio-chemical reactions require machines and chips (see figure below) including types for Gram positive, Gram negative, anaerobic bacteria, etc, and identify bacteria. However, these two methods detect mainly in protein levels, require expensive machines and cannot provide the antibiotic-sensitivity information making us back to the process of incubation.
Quorum Sensing System in Gram negative Bacteria
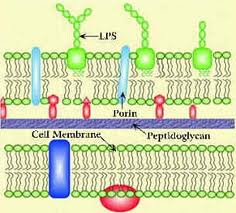
The cell wall of Gram-negative bacteria is composed of a relatively thin layer of peptidoglycan and an outer membrane which contains lipopolysaccharide (LPS). Due to its structure, quorum sensing molecules diffuse through the cell membrane and bind with intracellular receptors.
Generally speaking, quorum sensing system in gram negative bacteria functions as follows (Take LasI/LasR system for example):
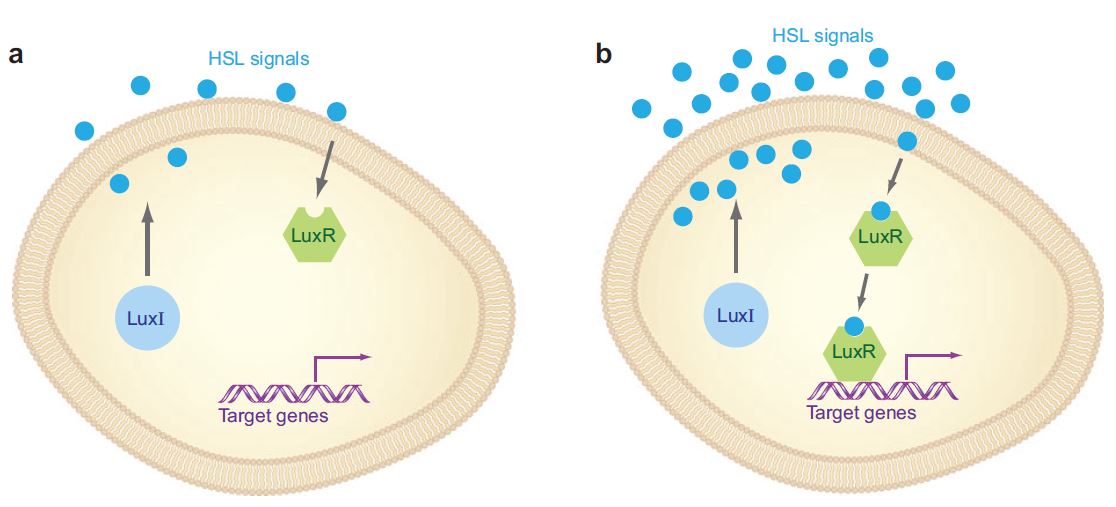
The LasI gene encodes an autoinducer synthase(LasI), and this autoinducer synthase produces quorum sensing molecules(autoinducers) called acyl-homoserine lactone(AHLs). Another gene LasR encodes for the response regulator of the autoinducers. Regulators bind with autoinducers and form complexes. They bind on target promoters, and then either activate or inhibit relevant down-stream genes.
Under low bacterial concentration, target genes are under minimum expression (Figure a above), but as bacterial concentration elevates, AHL molecules begin to bind with the intracellular response regulators and promote the expression of target genes, many of them related to bacterial virulence or biofilm formation. Therefore, there is a close relationship between bacterial resistance and quorum sensing. Besides having species-specific quorum sensing system, many species of gram-negative bacteria share a type of quorum sensing molecule, the AI-2(autoinducer-2) molecule.
PQS system in Pseudomonas aeruginosa
To date, it has been found that Pseudomonas aeruginosa has at least four quorum sensing systems: Las system (Las from e”las”tase), Rhl system (Rhl from “rh”amno”l”ipids), Qsc system (Qsc from “Q”uorum “s”ensing “c”ontroller ), and PQS system (PQS from “P”seudomonas “q”uinolone “s”ignal). In general, Las system is responsible for toxin expression; Rhl system is responsible for secretion system; Qsc system is as a negative regulator of Las system and Rhl system; PQS system is responsible for the virulence. The four systems also have interactions with each other.
Among these four systems, Las, Rhl and Qsc system all contain an AHL (acyl -homoserine lactone) activated receptor. Las system and Qsc system use 12-C AHL as QS (Quorum Sensing) molecules; on the other hand, Rhl system prefers to use 4-C AHL as QS molecules.
However, interestingly, PQS system is not activated through AHL, but a quinoline derivative: 2-heptyl-3,4-dihydroxyquinoline (also called “PQS”). Once PQS binds to pqsR, pqsR ─ as a transcriptional factor ─ can further activates two operons: pqsABCDE and phnAB (phn from "ph"e"n"azine). These two operons encode the enzymes that can synthesize PQS, and pqsE can catalyze PQS to become phenazine. Importantly, these phenazine products have been demonstrated to be involved in virulence of the organisms. For example, the phenazine pyocyanin production can enhance the ability of Pseudomonas aeruginosa to colonize the lungs of cystic fibrosis patients.
Reference
- Arul Jayaraman and Thomas K.Wood. Bacterial Quorum Sensing:Signals, Circuits, and Implications for Biofilms and Disease, Annu. Rev. Biomed. Eng. 2008. 10:145–67.
- Rutherford,et al. Bacterial quorum sensing: its role in virulence and possibilities for its control, Cold Spring Harb Perspect Med. 2012 Nov 1;2(11).
- Antunes LCM, et al. Quorum sensing in bacterial virulence, Microbiology(2010), 156, 2271–2282.
- Lo´ pez D, et al. Biofilms, Cold Spring Harb Perspect Biol 2010 ; 2:a000398.
- Jimenez PN, et al. The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa, Microbiol. Mol. Biol. Rev. 2012, 76(1):46.
- Kendra P. Rumbaugh. Quorum Sensing: Methods and Protocols, Methods in Molecular Biology, vol. 692.
- Jitesh A Soares and Brian MM Ahmer. Detection of acyl-homoserine lactones by Escherichia and Salmonella, Microbiology 2011, 14:188–193.
- Hsueh PR, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region, The British Infection Association 2011.
- Vanessa Sperandio. SdiA sensing of acyl-homoserine lactones by enterohemorrhagic E. coli (EHEC) serotype O157:H7 in the bovine rumen, Gut Microbes 1:6, 432-435; November/December 2010.
- Dubern, et al. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species, Mol Biosyst (2008) vol. 4 (9) pp. 882.
- Diggle, et al. 4-quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives, Int J Med Microbiol (2006) vol. 296 (2-3) pp. 83-91.
- Steindler, et al. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors, FEMS Microbiol Lett (2007) vol. 266 (1) pp. 1-9.
 "
"